Abstract
Background: Gallbladder cancer (GBC) has an extremely poor outcome. The aim of this study was to examine trends in GBC incidence, treatment and overall survival in a complete population of affected persons in a well-defined region in Sweden in 2000–2014.
Material and methods: Altogether 546 individuals with GBC were identified at Sweden’s Regional Cancer Centre West. Subjects were grouped into three 5-year periods (Period A: 2000–2004, Period B: 2005–2009 and Period C: 2010–2014) and the survival, diagnosis, staging, grading and treatment for each period were investigated. Patients dead at date of diagnosis (n = 39) and patients with not invasive cancer (n = 25) were not included in the analysis.
Results: The incidence was unchanged over the study period. The survival curves for the time periods were not significantly separated. Median survival was 4.7 months in Period A, 4.8 months in Period B and 6.1 months in Period C. Stage migration to more M1 in Periods B and C occurred and survival was improved for these cohorts. More individuals were diagnosed using only diagnostic imaging (p = .02). There were 177 curatively aiming operative procedures carried out on 482 persons (37%). The survival after surgery for the three periods improved over time (p = .02). Individuals who underwent a liver bed resection after a cholecystectomy had better survival than individuals who had cholecystectomy combined with liver resection. More persons were treated with chemotherapy, but no significant impact was found on survival in the total GBC population.
Conclusions: Although there were signs of improved diagnosis of GBC, the survival rate did not improve over time. There was a significant stage migration to more M1 in Periods B and C. Therapeutics able to downsize a cancer and increase the effectiveness of surgery with curative intent are warranted.
Introduction
Gallbladder cancer (GBC), while being the most common and aggressive malignancy of the biliary tree tract, is an uncommon disease in most parts of the world, with a low incidence rate compared with other cancers [Citation1]. In Sweden, with a population of 10 million, approximately 200 new cases are registered annually. Of these, 75% are older than 65 years. The female–male ratio in GBC is 2:1. During the last 10 years the incidence of GBC has been stable both in Sweden [Citation2] and in the United Kingdom [Citation3].
Gallbladder cancer is an extremely lethal disease. Only 10% of all affected persons are diagnosed at a stage amenable to surgical resection. Survival of patients with surgical management of GBC did not improve during the period 1991–2005 [Citation4]. It was reported that in a selected population, improvements in survival during the twenty-first century were attributable to aggressive radical surgery and improved surgical techniques [Citation5].
There is a large variation in reported survival figures. A Swedish article about GBC noted that median survival was 3.5 months after diagnosis [Citation6]. A recent overview of rare cancers in the UK reported 1- and 5-year survival rates of 50% and 21%, respectively [Citation3].
Magnetic resonance imaging (MRI) has been established as the best method for accurate staging of GBC [Citation7,Citation8]. The minimum staging evaluation of patients with suspected or proven GBC includes contrast cross-sectional imaging [Citation9]. In cases where surgery with curative intent is an option, a staging laparoscopy is recommended [Citation9].
Surgery is the only curative treatment for GBC patients [Citation9]. In patients with stages T1b and T2, this involves a primary resection of the gallbladder including en bloc resection of adjacent liver parenchyma. Patients with T1b, T2 or T3 disease that is incidentally identified in a cholecystectomy specimen should, after adequate staging, undergo secondary surgery with liver bed resection and complete regional lymph node resection. In a recent systematic overview [Citation10], the value of concomitant bile duct resection was assessed as unclear.
Based on a randomized phase III ABC-02 trial, the combination regimen of cisplatin plus gemcitabine (GEM) is considered the standard palliative therapy for patients with GBC [Citation11]. In the recently published BILCAP study with 447 patients, it was shown that treating patients with biliary tract cancer with capecitabine after surgery extended survival by a median of 15 months compared to surgery alone [Citation12].
To evaluate the impact of new cancer treatments, the mortality of all individuals with a defined diagnosis from a defined population must be included in an analysis. A survival analysis also has to include all non-interventional cases. First when an improved survival is observed in the whole cohort affect by a disease is it possible to claim that an interventional treatment has an effect on that disease.
Further subgroup analysis has to find the cause of these improvements. Despite the disadvantage with multiple uncontrolled factors, observational studies can provide information concerning overall survival and effectiveness of diagnosis and treatment among those with cancers in whom randomized trials are impossible.
From the start of this millennium, diagnostic and treatment efforts were successively centralized to the regional liver cancer center. Regional guidelines for GBC with multiple disciplinary therapy conferences that included more standardized staging were introduced in 2007. A national clinical hepatobiliary cancer register with detailed clinical data was started in Sweden in 2008.
The aim of the present analysis was to estimate overall survival of all individuals with GBC in a well-defined geographical region of Sweden in 2000–2014 and to analyze what diagnostic procedures and therapies that could be of relevance when analyzing outcomes, including overall survival.
Material and methods
The analyses were done in Gothenburg, Sweden, at the Transplant Institute at Sahlgrenska University Hospital and at the Regional Cancer Centre (RCC) West. From 2000 to 2014, a total of 546 individuals were diagnosed as having GBC and reported to the RCC in western Sweden.
Information concerning date of diagnosis, date of death, information about any histopathologically verified or clinically based diagnosis and TNM classification was retrieved from the RCC register. Therapy and additional information was obtained by reviewing patient charts. Patients in whom no surgical or specific anti-cancer therapy was documented were reported as having been given best supportive care (BSC).
Swedish pathologists are required to report all cases of morphologically defined cancer, including cancer in situ and adenoma and dysplasia, to the RCC. Where a clinician has established a cancer diagnosis, this also needs to be reported to the RCC register. The reported data need to include the stage of a cancer, based on clinical and imaging findings which are then classified according to TNM criteria (TNM 6 or higher).
In this study, to explore whether there was a continuous progress in outcome, data were divided into three equally long cohorts, Period A: 2000–2004, Period B: 2005–2009 and Period C: 2010–2014.
Statistics
Survival time was calculated from the date a diagnosis was established histopathologically or only clinically. The shortest observation time for survivors was 24 months or until death for all individuals. Survival curves were made using the Kaplan–Meier method and compared using the log-rank test.
Univariable and multivariable Cox proportional hazard regression analyses were used to select independent predictors for overall survival and Stata statistical software, release 15.1, was used [Citation13]. Multiple imputation has been used to impute values for stage in the multivariable Cox regression.
Other statistics were calculated using StatView software version 5.0 (SAS Institute, Cary, NC, USA) and Grand Pad Prism version 6.0 (GraphPad Software Inc., San Diego, CA, USA).
Ethics
The study was carried out in compliance with the Declaration of Helsinki principles.
The design and performance of the study were approved by the Regional Ethics Committee in Gothenburg (D 476-15).
Results
The incidence of GBC per 100,000 inhabitants in the western Sweden was 2.11 in 2000–2004 (Period A) and 2.35 in 2010–2014 (Period C). The number of individuals entered into the registry as having GBC was 182 (Period A), 168 (Period B) and 196 (Period C).
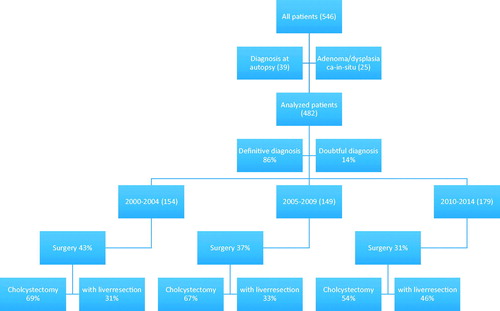
A flowchart presenting the variety of treatments in patients during the three time periods is depicted in .
The patients’ age and gender are given in . Twenty-three per cent of the patients were >80 years of age. The female/male ratio was 2.6:1.
Table 1. Demographics, staging and grading of the patients.
In 39 individuals, the diagnosis was established postmortem without preceding medical care. The relative numbers of individuals diagnosed postmortem declined from Period A (13%) to Period C (2%) (p = .001) (). These cases were excluded from further analysis. Of the remaining subjects, 487 were reported as International Classification of Diseases (ICD) code 23.9 and 20 patients were classified under other, varied ICD codes.
In 25 patients, the cancers were not invasive (adenoma/dysplasia n = 14, cancer in situ n = 11), why the analysis is restricted to 482 individuals with invasive GBC.
When scrutinizing available information, the diagnosis was given as ‘doubtful gallbladder cancer’ in 66 individuals (13%). Among these 66 individuals, there were 49 perihilar (PHC), 14 intrahepatic (IHC) and three other gastrointestinal (GI) cancer, alternatively GBC. These 66 individuals with doubtful GBC diagnosis were evenly distributed over the three periods (p = .83) ().
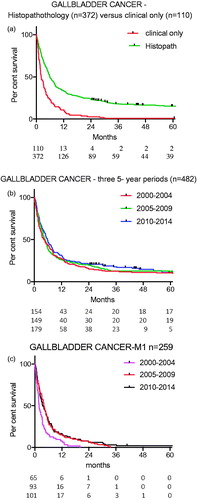
The relative percentage of patients in whom the diagnosis was established clinically only, mainly through computed tomography (CT) or MRI and without histopathology (n = 110), was 20% in Period A, 17% in Period B and 30% in Period C (p = .02, ).
The median survival for patients with a not histologically confirmed GBC was 2.2 months. Survival was higher when GBC was histopathologically confirmed (p < .0001) ().
Figure 2. (a) Survival for individuals whose diagnosis of GBC was histologically verified or based on clinical findings (p < .0001). (b) Survival over three time periods (p = .28). (c) Survival for individuals with stage M1 cancer. Period A versus Period B (p = .0005).
There were 101 available tumor specimens where the pathologist did not report any histopathological grading. Further, 11 tumors were reported as having squamous cell histology without reporting any grading. Of the 257 cases where tumor differentiation was reported, no grading migration throughout the three periods was identified ().
The percentage of patients without adequate staging declined from 15% in Period A to 2% in Period C (p < .0001) (). These 35 unstaged patients had a median survival of 2 months and their survival curve was super-imposed to the curve for patients with M1 (p = .91). Among those patients for whom clinical stage N1 (n = 59) or M1 (n = 259) was established, no T stage was reported. In six cases, the T stage was indeterminate called T? in .
In the population with a cancer that was staged (n = 447), there was a significant increase in metastatic disease (M1), from 49% in Period A to 66% in Period B (p = .0053). In total, 58% had M1. In 20 cases (4%), the tumors were T1, and in 35 (8%), T2 ().
The median survival was 5.5 months for those alternatively diagnosed as having IHC and 4.7 months for those likely having PHC cancer. The survival for patients with a defined, infiltrating GBC was borderline better compared with patients in whom the diagnosis of GBC was uncertain (p = .069).
The survival curves for 482 individuals with GBC for the three periods were not significantly separated (p = .28) (). Survival for more than 5 years was 10% for Period A and 12% for Period B. Period C had not ended at the time this article was prepared (shortest observation time 24 months) but five-year survival was projected to 12%. The univariate Cox regression analysis did not show any difference between Period A, B and C. In the multivariate Cox regression analysis, there was no significant difference between Periods A and B (p = .47). For Period C versus Period A, there was a borderline improved survival (p = .064) ().
Table 2. Uni- and multivariable Cox regression analyses on survival.
For patients with systemic metastatic disease (M1), survival curves were different. The survival in Period B was improved compared with Period A (p = .0005). The survival rate in Periods B and C was not different (p = .70) ().
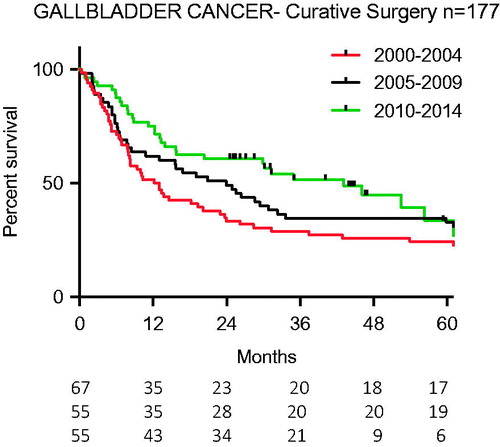
There were 177 curatively aiming operative procedures carried out on 482 persons in this study (37%). Diagnostic laparoscopies and palliative procedures (gastro-enterostomies and bilio-digestive fistulas) (n = 50) were not included in this total. There was no difference over time in the number of curatively aiming procedures but the p value was approaching statistical significance (p = .076). In 21 out of 90 (23%) cholecystectomies laparoscopic technique was used with no change over time (p = .54) (). The survival curves for the three periods were improved with time (p = .02) ().
Table 3. Surgery with curative intent, by type of surgery.
In the univariate analyses, the survival was 44% better in Period C compared to Period A (p = .016, ). But this finding was not significant in the multivariate analysis (p = .58).
Table 4. Uni- and multivariable Cox regression analyses on survival of patients undergoing surgery with curative intent (n = 177).
A complementary liver bed resection after a median 3 ± 1.1 months’ interval after initial cholecystectomy was carried out in 38 patients (). In another nine individuals scheduled for primary and 18 for secondary liver bed surgery, this procedure was discontinued because of intra-abdominal metastatic disease.
In total, 75 patients in the study had liver bed and, eventually bile duct resections (). The relative number of patients undergoing these combinations of surgery increased between Period B and Period C (p = .01).
Twenty-seven percent of the cholecystectomies were combined with liver resection. There was no difference in survival curves after liver bed resection between the three periods (p = .49).
Those individuals who had liver surgery done separately had significantly better survival rates, calculated from date of diagnosis, compared with those who had a cholecystectomy and concomitant liver resection (p = .02).
Of 20 patients with stage T1 GBC, three had concomitant and eight secondary liver surgery. All except one (91%) survived for more than 3 years. The remaining nine patients in this group were operated with open cholecystectomy (n = 7) and laparoscopic cholecystectomy (n = 2). Six (100%) have survived for more than 36 months (two have not yet reached the 36-month observation time). There was no significant difference in survival between T1 and T2 cancers (p = .5), but the limited number of patients in these groups makes this statement very uncertain.
Of 35 patients staged T2, 10 had concomitant and nine had secondary liver bed surgery. Ten were subjected to only cholecystectomy and six were not subjected to curatively aiming surgery. More than 3 years’ survival was recorded in 79%. Of the T2 patients treated with only cholecystectomy, 50% survived more than 3 years.
Bile duct resection and lymphadenectomy were, in the delayed surgery group, added to liver bed surgery in 12 out of 38 (32%) and as concomitant surgery in six of 37 (16%) patients (). The survival curves after liver resection with or without bile duct resection were not separated (p = .76).
In the group of individuals with T2N0M0 and T3N0M0, the survival was better after a cholecystectomy with liver bed surgery (n = 26) than after only a cholecystectomy (n = 21) (p = .002).
Fifty-nine persons were classified as N1; 12 had a concomitant liver bed resection and eight had a delayed liver bed resection with lymphadenectomy. Eight of these 19 (40%) survived more than 3 years.
In 177 patients, the surgery was performed with curative intent and of those surgery was defined as radical (R0) in 60 (35%). There were more R0 resections in Period B (43%) versus Period A (23%, p = .014). The 5-year survival after R0 resection was 65% and the survival difference between R0 and R1 resection was evident (p < .0001).
Regarding the time between primary and complimentary surgery, no difference in survival was identified in patients with an interval of less than 2.2 (mean 1.8 ± 0.4) months (n = 13) between procedures versus patients in whom the interval was longer than 3.2 (mean 4.2 ± 0.6) months (n = 14, p = .88). These intervals, less than 2.2 months versus more than 3.2 months were selected ad hoc in order to find two groups with a clear difference in interval.
There were 59 individuals surviving less than 30 days. This number decreased from 15% in Period B to 8% in Period C (p = .048).
Of the 482 patients, the 60 subjected to a curative procedure and the four post-op. dead never were actual for chemotherapy. Of the remaining 418, it was reported that 82 had chemotherapy and 15 COX-2 inhibitors.
There was no standard chemotherapy but treatment mainly included GEM. COX-2 was used in a clinical trial for persons with advanced GBC during the first 5-year period of the study (Period A). For these patients (n = 15), the median survival was 5.0 months [Citation11].
Palliative chemotherapy was used more frequently over time (Period A: 27/138 (20%), Period B: 25/125 (20/%), Period C: 45/155 (29%)) (Period A plus Period B versus Period C p = .03).
Three hundred and twenty one patients did not receive chemotherapy due to factors that contributed negatively to chemotherapy; high age and/or other diseases or too advanced cancer.
Median survival for those with advanced cancer who were given chemotherapy was 6.4 months. The indication for chemotherapy was metastatic or locally inoperable disease and recurrent disease. No adjuvant chemotherapy was given during the time studied. The survival over time were not different between the three periods studied (p = .58, ).
Figure 4. Survival after palliative chemotherapy over time and compared with patients given COX-2 (p = .40 and p = .74, respectively).
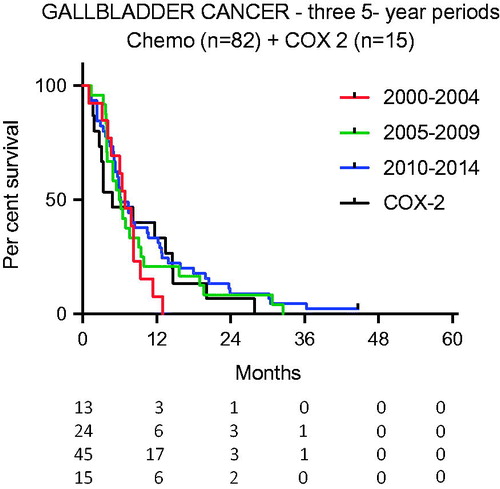
The survival rate for COX-2-treated individuals did not differ from the survival rate of those receiving active palliative chemotherapy (p = .74, ).
The survival curves for patients given chemotherapy versus patients receiving BSC were statistically separated (p < .0001). This statistical separation remained when patients surviving less than one month were excluded.
Discussion
During 2000–2014, there was a decrease in the number of individuals who had a postmortem diagnosis of GBC. The decreased number of postmortem diagnosis was related to more and better diagnostic imaging in the clinic. The minor increase in the incidence of GBC over the 15-year study period was likely a consequence of improved reporting to the register. It could also have been influenced by better and earlier diagnoses through improved radiology. It was observed that more patients during the last period had a diagnosis established without histopathologic confirmation. There was also an increasing number of patients with systemic metastases (M1), in Periods B and C versus Period A. That was evidently related to improved staging with CT and/or MRI, which then contributed to significantly better survival curves for these patients in Periods B and C. The Will Rogers phenomenon then occurred, i.e., moving an element from one set to another set raised the average value of both sets. This most likely explains the better survival for M1 patients seen in the later time periods ().
The outcome for the total population was poor, with an overall median survival of 5.0 months. The present analysis failed to identify a significant improvement in overall survival as the survival curves for the three time periods were not significantly separated (). In the multivariate analyses, the survival was not significantly better than in Period C than in Period A (p = .064, ). Subgroup analyses may be more interesting from a clinical point of view but are hampered by the low number of patients in the groups. In a subgroup analysis excluding patients without documented invasive cancer, i.e., cancer in situ and T0, the 5-year survival for Periods A and B was calculated to 10% and 12%, respectively. These results are in congruence with one study that reported no significant improvement in overall survival between 1991 and 2005 [Citation4].
The 5-year survival after R0 surgery was 65%, which is consistent with international reports on GBC [Citation1]. It has to be emphasized that a significant shift to more radical surgery was not identified.
The establishment of a multidisciplinary diagnostic and therapeutic board and the adoption of European Association for the Study of the Liver (EASL) guidelines for noninvasive diagnostics during Period B (2005–2009) created an opportunity for diagnostic improvements. Yet despite this, there was no change in the number of questionable diagnoses (n = 66) over time. Most patients with an uncertain GBC diagnosis or intrahepatic or perihilar bile duct cancer had such advanced cancer that an exact diagnosis could not be readily established. Consequently, they had a worse prognosis and their survival was short.
Twenty-two percent of the genuine cholecystectomies were performed using a laparoscopic approach. This figure did not change over time. When preoperative signs of GBC are detected, some surgeons advocate immediate conversion to radical resection, including port sites. No guidelines have so far been established [Citation1]. This strategy was followed in 37 out of 162 cholecystectomies that included concomitant liver resection and in 38 where the liver resection was performed in a second surgery. There was an impact on survival curves from date of diagnosis in favor of delayed liver resection. Those with delayed surgery are likely much less advanced, not diagnosed pre-surgically whereas those having concomitant liver bed removal must have been diagnosed ahead.
A 5-year survival rate of 60–100% has been reported elsewhere in individuals with T1b tumors, treated using a resection of the gallbladder liver bed and a lymph node dissection of the hepatoduodenal ligament [Citation14]. Other studies have not found any improved outcome in patients with pT1b tumors after radical liver bed resection [Citation15]. A systematic review concluded, albeit with a very low certainty of evidence, that also in patients with pT1b tumors there was a benefit from performing liver resection [Citation10]. A 3-year survival rate of almost 100% in the present study supports the view that simple cholecystectomy is sufficient for T1 cancers.
It has been proposed elsewhere that tumors that invade the subserosal layer (T2) should be considered for curative surgery: a liver bed resection and lymph node harvesting in the ligament [Citation12]. This can, however, be questioned, as there is increased morbidity. But in patients with T2 and T3 cancer, there was significantly better survival when cholecystectomy was combined with liver bed resection. Patients with N1 cancer had, in this analysis, a 42% 3-year survival after liver bed surgery and lymph node dissection. These individuals should therefore be considered for extensive surgery.
Time to recurrence is generally rapid, with a mean time of 4 months [Citation16]. The ideal time frame between cholecystectomy and secondary liver surgery for incidentally discovered GBC remains poorly defined. The majority of patients in this study were referred from local hospitals in the region. Due to limited surgical capacity, normally three months passed before those referred started receiving care from the receiving liver cancer center. It has been reported that delay due to a referral to a liver cancer service after cholecystectomy is not a risk factor for the disease becoming inoperable [Citation17]. It has, however, been suggested that re-resection should be performed within 10 days of the initial surgery [Citation12]. In this study, the consequence of the longer interval was not identified in the survival curves.
The classical study by Glimelius et al. stated already in 1996 that chemotherapy (5-fluorouracil/leucovorin and etoposide) can add prolonged high quality of life for a minimum period of 4 months in patients with advanced pancreatic and biliary cancer, but careful selection before and close monitoring during treatment are necessary [Citation17]. Gemcitabine, most often combined with cisplatin, is now considered as first-line therapy for patients with recurrent/advanced biliary tract cancer that gives progression-free survival for 3.4–6.4 months.
In this study, an increasing number of individuals with advanced disease were treated with chemotherapy, as described by Valle et al. [Citation18]. Also, a greater proportion of patients were given chemotherapy in the last period. Despite this increase, no significant impact on overall survival was seen in the last period. It should be noted that COX-2 treatment used in 15 patients in a clinical trial during Period A resulted in a survival curve not significantly separated from survival curves of those given chemotherapy [Citation11] ().
The COX 2-patients were selected study patients and therefore a survival comparison with non-study patients could be biased. That study showed that COX-treatment offered beneficial metabolic effects to weight-losing cancer patients by attenuation of resting metabolism and improved appetite due to decreased systemic inflammation.
As only a small proportion of the cohorts were treated with chemotherapy, even in the last time period, a possible impact on survival cannot be excluded.
There is no solid evidence regarding pre- or post-operative adjuvant chemotherapy in GBC.
A meta-analysis of published communications during 1967–2014 involving 3191 publications showed a non-significant improvement with adjuvant chemotherapy versus surgery alone [Citation19]. The value of adjuvant chemotherapy in biliary tract cancers was recently documented in phase III randomized clinical trial with 447 patients which showed that treating the disease with capecitabine after surgery extended survival by a median of 15 months compared to surgery alone [Citation12].
The conclusion, based on the present analysis of more than 500 individuals in a Swedish population with GBC split into three 5-year periods since 2000, is that there has unfortunately been no significant improvement in overall survival. The diagnosis of GBC may admittedly be difficult, especially in patients with concurrent cholecystitis or advanced disease. As the primary location in the biliary tree was questionable in 66 patients and 109 had a diagnosis without histopathological confirmation, the results of this analysis should be interpreted with caution. The incomplete staging and grading make comparisons between subgroups difficult but as more than half of the patients with GBC had M1 this concern is reduced.
For patients with a T1 tumor, cholecystectomy is adequate. For patients with GBC staged T2–T3, extensive surgery is of value but a clinical trial is needed to determine which treatment strategy is the most effective. Therapeutics with the capacity to downsize GBC and thereby to increase the number of candidates for surgery with curative intent is warranted.
Acknowledgments
The Director, Professor Nils Konradi, and Assistant Director, Susanne Amsler-Nordin of the Regional Cancer Centre West, Sweden have generously supported this analysis. All surgeons at the Transplant Institute, Sahlgrenska University Hospital, Gothenburg, Sweden, are acknowledged for their excellent clinical work with study subjects. The English was reviewed and corrected by Mr Jonathan Stubbs, professional editor of English medical articles and Proper English AB.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Kanthan R, Senger JL, Ahmed S, et al. Gallbladder cancer in the 21st century. J Oncol. 2015;2015:967472.
- National Quality Registry for Liver, Bile Duct and Gallbladder Cancer (SweLiv), Årsrapport nationellt kvalitetsregister; 2016. Available from: https://www.cancercentrum.se/globalassets/cancerdiagnoser/lever-och-galla/kvalitetsregister/sweliv_rapport_2015_final.pdf
- Cancer Research UK. Gallbladder cancer. Available from: www.cancerresearchuk.org
- Mayo SC, Shore AD, Nathan H, et al. National trends in the management and survival of surgically managed gallbladder adenocarcinoma over 15 years: a population-based analysis. J Gastrointest Surg. 2010;14:1578–1591.
- Zhu AX, Hong TS, Hezel AF, et al. Current management of gallbladder carcinoma. Oncologist. 2010;15:168–181.
- Shabo I, Nordenskjold K, Svanvik J. The incidence of gallbladder carcinoma in Sweden has decreased – the poor prognosis might possibly be improved through radical surgery. La¨kartidningen. 2001;98:4584–4589.
- Hennedige TP, Neo WT, Venkatesh SK. Imaging of malignancies of the biliary tract – an update. Cancer Imaging. 2014;14:14.
- Kim SJ, Lee JM, Lee ES, et al. Preoperative staging of gallbladder carcinoma using biliary MR imaging. J Magn Reson Imaging. 2015;41:314–321.
- Aloia TA, Járufe N, Javle M, et al. Gallbladder cancer: expert consensus statement. HPB (Oxford). 2015;17:681–690.
- Sternby Eilard M, Lundgren L, Cahlin C, et al. Surgical treatment for gallbladder cancer – a systematic literature review. Scand J Gastroenterol. 2017;52:505–514.
- Lundholm K, Daneryd P, Bosaeus I, et al. Palliative nutritional intervention in addition to cyclooxygenase and erythropoietin treatment for patients with malignant disease: effects on survival, metabolism, and function. Cancer. 2004;100:1967–1977.
- John Neil P, Richard F, Daniel H, et al. Adjuvant capecitabine for biliary tract cancer: the BILCAP randomized study. Abstract 4006 ASCO; 2017.
- StataCorp. 2017. Stata: release 15. Statistical software. College Station (TX): StataCorp LLC.
- Yi X, Long X, Zai H, et al. Unsuspected gallbladder carcinoma discovered during or after cholecystectomy: focus on appropriate radical re-resection according to the T-stage. Clin Transl Oncol. 2013;15:652–658.
- Hu L, Wang B, Liu X, et al. Unsuspected gallbladder cancer: a clinical retrospective study. J Breast Cancer. 2013;16:631–635.
- Kaushik SP. Current perspectives in gallbladder carcinoma. J Gastroenterol Hepatol. 2001;16:848–854.
- Glimelius B, Hoffman K, Sjödén PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7:593–600.
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281.
- Ma N, Cheng H, Qin B, et al. Adjuvant therapy in the treatment of gallbladder cancer: a meta-analysis. BMC Cancer. 2015;15:615.