Abstract
Background: Upon FDA/EMEA registration for hepatocellular carcinoma (HCC), sorafenib received a broader therapeutic indication than the eligibility criteria of the landmark SHARP trial. This allowed treatment of SHARP non-eligible patients in daily clinical practice.
Aim: To assess sorafenib efficacy and safety in SHARP eligible and non-eligible patients, and determine the validity of the current therapeutic indication as described by the FDA/EMEA.
Patients and methods: Consecutive patients treated with sorafenib for advanced HCC at two Dutch tertiary referral centers between 2007 and 2016 were analyzed retrospectively. Primary outcome was overall survival (OS). Secondary outcomes were time to progression (TTP), response rate, adverse events and reasons for discontinuation. Outcomes were compared between SHARP eligible and non-eligible patients.
Results: One hundred and ninety-three of 257 (75%) patients were SHARP eligible. SHARP eligible patients (9.5 months, 95% CI 7.7–11.3) had a longer median OS than non-eligible patients (5.4 months, 95% CI 3.6–7.1) (log-rank p < .001). SHARP non-eligible patients were more often Child–Pugh B, had higher AST and ALT levels and developed more grade 3–4 liver dysfunction (44 versus 23%, p < .001) during treatment. SHARP ineligibility remained the strongest predictor of OS (HR 1.78, 95% CI 1.32–2.41) and an independent predictor of TTP (HR 1.45, 95% CI 1.05–2.00) in multivariable analysis.
Conclusions: Landmark trial outcomes of sorafenib for HCC are reproducible in daily practice, provided that the SHARP eligibility criteria are respected. Based on the findings of this and previous studies, sorafenib usage should be restricted to Child–Pugh A patients.
Introduction
For almost a decade, the multikinase inhibitor sorafenib has been the only registered treatment for advanced hepatocellular carcinoma (HCC). Implementation of sorafenib as standard treatment for advanced HCC is based on the results of two randomized phase III trials: the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) trial and the parallel Sorafenib Asian-Pacific (AP) trial [Citation1,Citation2]. Both studies demonstrated a sorafenib survival benefit of 3 months compared with placebo in strictly selected patients. HCC develops mostly in patients with liver cirrhosis, thus only patients with well-preserved liver function (Child–Pugh A) were eligible for study participation. Presence or absence of underlying cirrhosis was not specified. Accordingly, the current guidelines recommend sorafenib treatment for advanced HCC in patients with Child–Pugh A liver function only, without specifying the presence or absence of underlying cirrhosis [Citation3,Citation4]. Nonetheless, the Food and Drug Administration (FDA) and the European Medicines Evaluation Agency (EMEA) gave sorafenib a broader therapeutic indication than the eligibility criteria of the landmark SHARP trial [Citation5]. The guideline endorsed Barcelona Clinic Liver Cancer (BCLC) staging system does not exclude Child–Pugh B patients for sorafenib treatment [Citation6]. Consequently, several real-life studies showed that sorafenib is currently prescribed to a broad spectrum of patients, including substantial numbers (12.5–34%) of Child–Pugh B patients [Citation7–16]. It is well-established that patients with Child–Pugh B liver function have significantly poorer outcomes, although sorafenib toxicity seems not related to Child–Pugh status [Citation9,Citation12,Citation15]. Still, in absence of randomized-controlled trials, the exact benefit in patients not-meeting the SHARP eligibility criteria, specifically in Child–Pugh B patients, remains controversial. Real-life studies in some tumor types (i.e., colorectal and prostate cancer) have shown decreased outcomes when systemic therapies are given outside the landmark trial eligibility criteria [Citation17,Citation18], whereas outcomes were comparable in patients with bile tract cancer [Citation19]. A direct comparison between SHARP eligible and non-eligible subgroups in advanced HCC has not been conducted. Hence, it remains unknown whether sorafenib treatment of a substantial numbers of SHARP ineligible patients, which are exposed to sorafenib toxicity and results in significant healthcare costs, is beneficial. Hence, this retrospective multicenter study aims to assess the real-life efficacy and safety of sorafenib in advanced HCC, comparing SHARP eligible and non-eligible subgroups; and to determine whether the current sorafenib therapeutic indication as described by EMEA/FDA is valid based on the current evidence.
Patients and methods
Study population
All consecutive patients, ≥18 years old, evaluated at two tertiary HCC referral centers in the Netherlands [Academic Medical Center (AMC), Amsterdam, and Erasmus University Medical Center (EMC), Rotterdam] who received at least one dose of sorafenib for advanced HCC between May 2007 and December 2016 were included in this retrospective study. Study-based sorafenib was allowed, but patients who received study-based selective internal radio-embolization therapy (SIRT) combined with sorafenib were excluded from this analysis [Citation20].
HCC diagnosis was established histologically or by the American Association for the Study of Liver Diseases (AASLD) imaging criteria [Citation21]. Absence or presence of underlying cirrhosis was established on criteria proposed by Mittal et al. [Citation22]. Patients were staged by four-phase computed tomography (CT) or dynamic contrast-enhanced magnetic resonance imaging (MRI) and discussed at a multidisciplinary meeting. Patients were considered for treatment with sorafenib according to the BCLC guidelines [Citation3]. HIV infection was not a contraindication for treatment. Following the indications provided by the manufacturer, patients received sorafenib 400 mg twice daily (BID), but were allowed to receive lower starting doses (200 mg twice daily), toxicity-adjusted dosing and treatment interruptions at the discretion of the medical oncologist to cope with drug-related adverse events [Citation5]. The Institutional Review Board (IRB) of the AMC (reference number W17_420#17.488) approved the study and a waiver for informed consent was given.
Data collection
Potential patients were identified using keywords and diagnostic codes from the electronic patient record and pharmacy records. Included keywords were ‘hepatocellular carcinoma’, ‘HCC’, ‘malignancy liver’, ‘liver neoplasm’ and ‘liver malignant neoplasm’. Medical records were reviewed and clinical data was manually extracted. Extracted data included previous treatments, baseline patient and tumor characteristics and treatment details including treatment emergent adverse events (AE), which was prospectively monitored as part of standard care. Efforts were made to collect missing data, for example by contacting referring hospitals for additional data on referred patients.
SHARP eligibility criteria
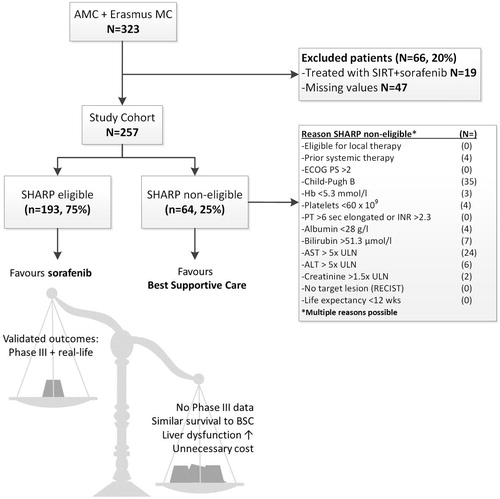
Subgroups were made based on meeting or not meeting the eligibility criteria of the SHARP trial [Citation2]. These criteria are shown in . In summary, patients were required to have advanced stage HCC or intermediate stage HCC which was not or no longer eligible for surgical or locoregional therapies, without previous systemic therapy for HCC. SHARP eligible patients were required to have an ECOG PS 0-2, Child–Pugh A liver function and adequate renal and hematological function. The actual life expectancy was not mentioned in the files, but was considered to be within the limits of the SHARP eligibility criteria (≥12 weeks) based on descriptive data. Patients violating one or more SHARP eligibility criteria were assigned to the ‘SHARP non-eligible subgroup and patients meeting all criteria were assigned to the ‘SHARP eligible’ subgroup. Patients who could not be assigned to a subgroup due to missing data were excluded from this study.
Outcome measures
The primary outcome, overall survival (OS), was calculated from the date of the first dose of sorafenib until death or censored on the last known date to be alive. Survival status was checked in the electronic medical record and verified by using the municipal records database on 17 January 2018.
Time to progression (TTP) was defined as the time from start of sorafenib treatment until clinical or radiological disease progression. Radiological response evaluation was performed every 2–3 months as part of standard of care and assessed by experienced abdominal radiologists using the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) [Citation23]. Progression-free patients were censored at the time of last radiological evaluation. Patients who died or were lost to follow-up before first radiological evaluation, were excluded from TTP analysis. In response evaluation, all patients who showed clinically progressive disease in the absence of radiological evaluation were considered to have progressive disease. All adverse events were classified according to the National Cancer Institute’s Common Toxicity Criteria (NCI-CTC v4.03) [Citation24]. Liver dysfunction was defined as occurrence or deterioration of hyperbilirubinemia, ascites or encephalopathy.
Statistical analysis
Continuous variables were expressed as medians and ranges, and categorical variables as absolute and relative frequencies. The Pearson’s chi-square or Fisher’s exact test where appropriate, were used to compare categorical data in the SHARP eligible and non-eligible subgroups. Continuous data was compared using the Student’s t-test or Mann–Whitney U-test, where appropriate. The Kaplan–Meier method and log-rank tests were used to estimate and compare OS and TTP curves. Cox proportional hazard regression analysis was used to calculate hazard ratios (HRs) and assess the association of baseline variables, including literature reported predictors, with OS and TTP. All predictors with a two-sided p value <.05 in univariate analysis were included in a subsequent multivariable model in which correlated predictors were adjusted to reduce overlapping variance. In order to identify the impact of the separate SHARP eligibility criteria, we performed an exploratory univariate analysis of the individual criteria separately. A two-sided p value <.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS statistics (version 23.0; IBM Corp., Armonk, NY).
Results
Patient characteristics
Between May 2007 and December 2016, a total of 323 patients with advanced HCC were treated with sorafenib. Of these, 66 patients (20%) were excluded due to incomplete data (n = 47) or because they received combined SIRT and sorafenib treatment (n = 19) (). The remaining 257 patients formed the study cohort of whom baseline patient and tumor characteristics are summarized in . The majority of patients had liver cirrhosis (79%), and 218 patients (85%) were in the Child–Pugh A class. The most common etiological factors were alcohol (34%), followed by hepatitis B virus infection (HBV; 16%) and hepatitis C virus infection (HCV; 16%). At the start of sorafenib treatment, most patients had advanced stage HCC (73%) and 93 patients (36%) received prior treatment for HCC, mainly TACE (21%). In total, 193 of the 257 (75%) were considered SHARP eligible. Reasons for non-eligibility of the other 64 patients are listed in . SHARP non-eligible patients had more often Child–Pugh B liver function with lower albumin and higher bilirubin, aspartate transaminase (AST) and alanine transaminase (ALT) levels. Less common reasons for SHARP ineligibility were prior systemic treatment for HCC (n = 4), low platelet count (n = 4) or low hemoglobin (n = 3). SHARP non-eligible patients were younger (63 versus 66 years, p = .040), but other baseline clinical and tumor characteristics were comparable between subgroups.
Table 1. Baseline characteristics of all, SHARP eligible and non-eligible patients.
Overall survival
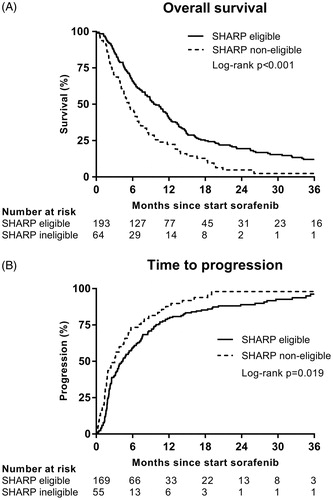
After a median follow-up of 45.8 months (range 0.2–63.2), 232 of 257 (90%) patients had died. The median OS for all patients receiving sorafenib was 8.4 months (95% CI 6.9–9.9). The median OS in the SHARP eligible subgroup was 9.5 months (95% CI 7.7–11.3) compared with 5.4 months (95% CI 3.6–7.1) in the SHARP non-eligible subgroup (log-rank p < .001; ).
Figure 2. (a) Overall survival and (b) time to progression in SHARP eligible and SHARP non-eligible subgroups. Thirty-three patients were not evaluable for TTP analysis.
In univariable Cox regression analysis (), not meeting the SHARP inclusion criteria was the strongest predictor of an increased risk of mortality [hazard ratio (HR) = 1.81, 95% CI 1.35–2.44]. Other baseline factors that were associated with an increased risk of mortality were ECOG performance status 2, tumor size >72 mm (above median), macrovascular invasion (MVI) and α-fetoprotein (AFP) ≥ 400 ng/ml, whereas patients that received prior HCC treatment had a better prognosis (HR 0.67, 95% CI 0.51–0.88). In subsequent multivariable analysis, not meeting the SHARP inclusion criteria (HR = 1.78, 95% CI 1.32–2.41), MVI (HR = 1.34, 95% CI 1.02–1.76) and AFP ≥400 (HR = 1.60, 95% CI 1.22–2.11) were independently associated with reduced survival. An additional exploratory analysis aimed at assessing the impact of the individual SHARP eligibility criteria (Supplementary Table S1), showed that Child–Pugh B (HR 2.03, 95% CI 1.40–2.95), hemoglobin <5.3 mmol/l (HR 13.85, 95% CI 4.16–46.06), bilirubin >51.3 µmol/L (HR 2.46, 95% CI 1.16–5.25), AST >5× ULN (HR 2.66, 95% CI 1.72–4.11), ALT >5× ULN (HR 1.18, 95% CI 0.52–2.67) and creatinine >1.5× ULN (HR 8.57, 95% CI 2.08–35.39) were associated with reduced OS. A multivariable analysis was not conducted due risk of collinearity of multiple violations, and limited sample size of subgroups.
TTP and response rate
Data on TTP () was available in 224 patients (87%); 33 patients (13%) died or were lost to follow-up before clinical or radiological disease progression. Overall, the median TTP was 3.8 months (95% CI 2.9–4.6). SHARP eligible patients had a significant longer TTP (4.3 months, 95% CI 3.1–5.6) than non-eligible patients (3.0 months, 95% CI 1.7–4.4) (log-rank p = .019). After correction for other univariable predictors (HBV, MVI and AFP ≥400), not meeting the SHARP eligibility criteria remained an independent predictor of poor TTP (HR 1.45, 95% CI 1.05–2.00) (Supplementary Table S2). The best radiological response according to RECIST 1.1 was not statistically different between eligible and non-eligible subgroups ().
Table 2. Treatment details and adverse events of SHARP eligible and non-eligible patients.
Table 3. Hazard ratios for overall survival in univariable and multivariable analysis.
Treatment details and adverse events
Treatment details and adverse events are summarized in . Median duration of sorafenib treatment was 13 weeks (range 0–225 weeks) with a median maximum-tolerated dose of 200 mg twice daily, reflecting that the majority of patients (59%) did not reach the maximum dose. The most common treatment emergent AE’s (all grades) were gastrointestinal (37%), liver dysfunction (35%), asthenia (30%) and skin toxicity (34%). The most common grade III/IV AE’s were liver dysfunction (28%), followed by gastrointestinal (13%) skin toxicity (11%). During sorafenib treatment, liver dysfunction occurred more frequently in the SHARP non-eligible patients (56 versus 28%, p < .001) compared with SHARP eligible patients. Non-eligible patients developed more often grade 3–4 liver dysfunction (44 versus 23%, p = .001), but maximum-tolerated sorafenib dose and AE’s were comparable between subgroups. Patients who were SHARP non-eligible showed a trend towards shorter treatment duration (9 versus 13 weeks, p = .052) and terminated treatment more often due to combined progression and AE’s (38 versus 22%, p = .030). In SHARP eligible patients, the main reason for permanent discontinuation was disease progression (54%).
Discussion
We showed that SHARP eligible patients treated in daily clinical practice had an OS similar to those treated in the SHARP trial (9.5 versus 10.7 months, respectively), whereas SHARP non-eligible patients had significantly reduced survival (5.4 months), reduced TTP and more liver dysfunction during treatment.
In our cohort, a minority of patients (25%) did not meet the SHARP eligibility criteria, mainly due to Child–Pugh class B liver function or serum AST/ALT values exceeding five times the upper limit of normal. This suggests that the observed survival difference, between SHARP eligible and non-eligible patients can be explained by more compromised liver function in the latter subgroup. Likewise, there was an increased occurrence of (severe) liver dysfunction whereas maximum-tolerated dose and other toxicity types were comparable. Hence, this study confirms the remarks made by the authors of both sorafenib landmark studies, which stressed that restricting enrollment to Child–Pugh A patients, could have potentially prevented deaths related to advanced liver disease masking the effects of sorafenib [Citation1,Citation2].
Despite these remarks and the guidelines advising sorafenib treatment of advanced HCC in Child–Pugh A patients only [Citation3], multiple cohort studies showed that prescription to Child–Pugh B patients has not been uncommon (12–44%, ) in the past decade [Citation9,Citation12,Citation15]. As sorafenib toxicity in these patients is comparable to Child–Pugh A patients, sorafenib seems a safe treatment option; however, safety alone is not enough to consider sorafenib as standard of care. Our study validates the findings of a series of previous studies, both retrospective and prospective (), showing that Child–Pugh B patients have a poor outcome on sorafenib treatment. Interestingly, the outcomes of SHARP non-eligible patients treated with sorafenib were inferior to historic cohorts of SHARP eligible patients receiving placebo (7.9 months) [Citation2] and comparable to patients with Child–Pugh B receiving best-supportive care (BSC) only (5 months) [Citation3,Citation25]. This suggests that sorafenib is non-superior to BSC in patients with more advanced liver disease. Despite the multitude of studies counting almost 5000 patients treated with sorafenib, including more than 1100 Child–Pugh B patients, the conclusions on the usage in these patients remain conflicting (). Ideally a randomized placebo-controlled trial should be conducted to determine the efficacy in Child–Pugh B patients. Unfortunately, such an initiative (BOOST trial) had to be terminated prematurely due to slow enrollment, demonstrating the difficulty in recruiting these patients and carrying out such a trial [Citation26]. Still, healthcare authorities increasingly demand validation of (expensive) drugs and both clinicians and patients are facing dilemmas when considering treatment. The significant costs of sorafenib therapy, which proved only to be cost-effective in patients with compensated liver disease and when applying proper dose adjustments, underlines the need of strict patient selection [Citation27,Citation28]. Therefore, we conclude that currently there is insufficient evidence for sorafenib treatment beyond the SHARP eligibility criteria, specifically in patients with Child–Pugh B liver function, and that the registered therapeutic indication and the BCLC staging system should restrict treatment to Child–Pugh A patients. The limitations of Child–Pugh A as a selection criterion and alternatives [i.e., Albumin-Bilirubin (ALBI) score] have been discussed previously [Citation8,Citation29]. Our findings support adherence to the current guidelines with careful patient selection and dose-adjustments according to the current evidence. This may reduce non-beneficial exposure of patients to sorafenib toxicity and the unnecessary healthcare costs.
Table 4. Studies comparing sorafenib outcomes in Child–Pugh A and B subgroups in advanced HCC.
Still, even in SHARP eligible patients further optimization of sorafenib usage is possible, indicated by low response rates, heterogeneity in survival outcomes and lack of a significant improvement in patient-reported quality of life variables [Citation2]. In the future, molecular biomarkers, i.e., tumor profiling or biochemical serum markers, might aid in further selecting the optimal candidates for sorafenib or future trials.
Our study has several limitations, including the retrospective design with its inherent drawbacks. Non-availability of key parameters (i.e., Child–Pugh classification) led to exclusion of some patients, as adherence to the SHARP eligibility criteria could not be assessed. Furthermore, the retrospective assessment of parameters that are subject to inter-observer variability, such as ECOG PS or life expectancy, might have caused inaccurate assessment. Strengths of our study include the focus on unselected patients in the daily clinical practice of two tertiary referral centers for HCC, covering roughly 30–40% of the Dutch HCC population. The construction of a robust dataset was possible due to the standardized evaluation of all patients in a multidisciplinary team meeting, prospective monitoring of drug toxicity and standardized RECIST 1.1 response monitoring.
In conclusion, our results confirm that the trial outcomes of sorafenib in advanced HCC are reproducible in daily clinical practice, provided that the SHARP eligibility criteria are respected. Based on our findings and the results of previous studies, sorafenib usage should be restricted to Child–Pugh A patients.
Supplemental Material
Download MS Word (73.5 KB)Disclosure statement
H.-J.K. is member of the advisory board for Ipsen and Sirtex, and received an unrestricted research grant from Bayer (no grant numbers apply). R.B.T. served as a speaker for Gore WL, Bayer, and Norgine, and is member of the advisory board for Gilead. R.d.M. served as a speaker for Norgine and as a consultant for Cook Medical. O.v.D. served as consultant for Cook Medical. All other authors have declared no conflicts of interest. The study was designed and conducted by academic investigators.
References
- Cheng A-L, Kang Y-K, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34.
- Llovet J, Ricci S, Mazzaferro V. Sorafenib in advanced hepatocellular carcinoma N Engl J Med. 2008;359:378–390.
- EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943.
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022.
- Bayer Schering Pharma AG. EU Summary of Product Characteristics for Nexavar, 21 July 2011, Germany.
- Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150:835–853.
- Abou-Alfa GK, Amadori D, Santoro A, et al. Safety and efficacy of sorafenib in patients with hepatocellular carcinoma (HCC) and Child-Pugh A versus B cirrhosis. Gastrointest Cancer Res. 2011;4:40–44.
- Edeline J, Blanc JF, Johnson P, et al. A multicentre comparison between Child Pugh and albumin-bilirubin scores in patients treated with sorafenib for hepatocellular carcinoma. Liver Int. 2016;36:1821–1828.
- Hollebecque A, Cattan S, Romano O, et al. Safety and efficacy of sorafenib in hepatocellular carcinoma: the impact of the Child-Pugh score. Aliment Pharmacol Ther. 2011;34:1193–1201.
- Iavarone M, Cabibbo G, Piscaglia F, et al. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54:2055–2063.
- DAF LG, Barroso-Sousa R, Bento AD, et al. Safety and efficacy of sorafenib in patients with Child-Pugh B advanced hepatocellular carcinoma. Mol Clin Oncol. 2015;3:793–796.
- Marrero JA, Kudo M, Venook AP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: the GIDEON study. J Hepatol. 2016;65:1140–1147.
- Ogasawara S, Chiba T, Ooka Y, et al. Sorafenib treatment in Child-Pugh A and B patients with advanced hepatocellular carcinoma: safety, efficacy and prognostic factors. Invest New Drugs. 2015;33:729–739.
- Pinter M, Sieghart W, Graziadei I, et al. Sorafenib in unresectable hepatocellular carcinoma from mild to advanced stage liver cirrhosis. Oncologist. 2009;14:70–76.
- Pressiani T, Boni C, Rimassa L, et al. Sorafenib in patients with Child-Pugh class A and B advanced hepatocellular carcinoma: a prospective feasibility analysis. Ann Oncol. 2013;24:406–411.
- Worns MA, Weinmann A, Pfingst K, et al. Safety and efficacy of sorafenib in patients with advanced hepatocellular carcinoma in consideration of concomitant stage of liver cirrhosis. J Clin Gastroenterol. 2009;43:489–495.
- Mol L, Koopman M, van Gils CW, et al. Comparison of treatment outcome in metastatic colorectal cancer patients included in a clinical trial versus daily practice in the Netherlands. Acta Oncol. 2013;52:950.
- Templeton AJ, Vera-Badillo FE, Wang L, et al. Translating clinical trials to clinical practice: outcomes of men with metastatic castration resistant prostate cancer treated with docetaxel and prednisone in and out of clinical trials. Ann Oncol. 2013;24:2972–2977.
- Dierks J, Gaspersz MP, Belkouz A, et al. Translating the ABC-02 trial into daily practice: outcome of palliative treatment in patients with unresectable biliary tract cancer treated with gemcitabine and cisplatin. Acta Oncol. 2018;57:807–812.
- Evaluation of sorafenib in combination with local micro-therapy guided by Gd-EOB-DTPA enhanced MRI in patients with inoperable hepatocellular carcinoma. J Clin Oncol. 2011;29:28023040.
- Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236.
- Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14:124–131.e1.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216.
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. US Department of Health and Human Services, 2010 [cited2018Jun7]. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/About.html
- Cabibbo G, Enea M, Attanasio M, et al. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274–1283.
- Sorafenib in first-line treatment of advanced B Child Hepatocellular Carcinoma (BOOST) [Internet]. U.S. National Library of Medicine (NLM) at the National Institutes of Health (NIH); [cited 2018 Jun 8]. Available from: https://clinicaltrials.gov/ct2/show/NCT01405573?term=Boost+trial+HCC&rank=1
- Camma C, Cabibbo G, Petta S, et al. Cost-effectiveness of sorafenib treatment in field practice for patients with hepatocellular carcinoma. Hepatology. 2013;57:1046–1054.
- Parikh ND, Marshall VD, Singal AG, et al. Survival and cost-effectiveness of sorafenib therapy in advanced hepatocellular carcinoma: an analysis of the SEER-Medicare database. Hepatology. 2017;65:122–133.
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558.