Abstract
Background: Generally, screen-detected cancers have more favourable tumour characteristics than clinically detected or symptomatic cancers. Less is known, whether the tumour characteristics of breast cancer have changed over time into more favourable in general and whether the changes have been similar in all ages.
Material and methods: The aim of this study was to explore the change of breast cancer characteristics in parallel to the implementation of modern diagnostic methods in three age groups over four 5-year time periods between 1992 and 2011. The data from 942 primary breast cancers in one university hospital district in Finland were combined with data from the Finnish Cancer Registry and the Mass Screening Registry. The association of favourable tumour characteristics with time period, age group and diagnostic methods was explored.
Results: The most discernible secular change was the increase in oestrogen (ER)-positive cancers in every consecutive time period. The risk for ER positivity in the second, third and fourth period was 2- to 2.71-fold compared to the first period. An increase in small tumours and node-negative tumours was detected during the most recent years of data collection. The secular changes were observed in all age groups; however, overall ER positivity was most frequent among women beyond screening age and small tumours among screening-aged women. The increase in small and node-negative tumours could partly be explained by the implementation of new radiological methods.
Conclusions: This study detected a secular change of tumour characteristics into more favourable irrespective of age group. If the trend continues, it seems that we are going to have a breast cancer population of mainly small ER-positive breast cancers in the future forcing to rethink the therapeutic approach.
Introduction
Population-based mammographic screening programmes were introduced in many European countries in the late 1980s [Citation1]. In Finland, nationwide screening for breast cancer was introduced in 1987, first as a population-based cohort study covering two-thirds of the Finnish municipalities [Citation2]. Since 1992, municipalities have been bound by law to offer biennial screening for women from 50 to 59 years of age. However, the screening policies have varied between the municipalities: many have also invited women from 60 to 64 years of age and a few women from 60 to 69 years of age. Based on the Government Decree on Screenings from 2007, the screening population has gradually been extended, and currently (2018), biennial screening covers women from 50 to 69 years of age in the whole country [Citation3].
After the introduction of population-based screening, the incidence, especially that of early breast cancer, has strongly increased [Citation4,Citation5]. In contrast to increasing incidence rates, breast cancer mortality rates have been modestly but steadily falling in most European countries over the past decades from an age-standardized mortality ratio of 21.3/100.000 in 1990 to 15.2/100.000 in 2012 [Citation6,Citation7]. In Finland, the age-standardized mortality ratio was 16.4/100.000 in 1983–1987 and 11.8/100.000 in 2015. During the past 10 years, the annual decline of breast cancer mortality has been 2.2% [Citation3].
The use of ultrasound as a supplemental imaging method to mammography as well as ultrasound-guided biopsies has improved staging and risk assessment of breast cancers [Citation8,Citation9]. In addition, digitalization has further increased detection rates of mammography in the twenty-first century [Citation10,Citation11]. Magnetic resonance imaging (MRI) as an adjunct method has improved diagnostic assessment among women with contradiction in conventional imaging, lobular histology and occult breast cancer [Citation12]. At the same time, general breast cancer awareness has increased and more opportunistic imaging is performed, further increasing the incidence of early breast cancer [Citation13].
Molecular subtypes of breast cancer are being defined and classified better in the twenty-first century than ever before. The 2011 St Gallen International Expert Consensus recommends detecting oestrogen (ER) and progesterone (PgR) receptor status, proliferation marker Ki67 and possible human epidermal growth factor (HER2) amplification and/or overexpression in addition to histology. Based on these procedures, four subgroups have been defined: luminal A, luminal B, HER2-positive and triple-negative breast cancer [Citation14]. These act as prognostic and predictive subgroups helping clinicians better understand the natural course of breast cancer and plan the therapeutic approach.
Studies have shown that screen-detected cancers have more favourable tumour characteristics than clinically detected or symptomatic cancers [Citation15–17]. However, less is known about whether the differences in tumour characteristics are attributable to diagnostic improvements and whether they are similar in all ages.
The aim of this study was to explore whether the breast cancer characteristics have shifted to more favourable among women of all ages around the beginning of the twenty-first century and whether there is an association between the possible changes and the parallel implementation of novel diagnostic methods in 1992–2011.
Patients and methods
In this study, the secular change of breast cancer tumour characteristics (size, nodal status, hormone receptor status, HER2 status and Ki67) and their correlation to employment of diagnostic methods (ultrasound [US], MRI and sentinel node biopsy [SNB]) were evaluated in three age groups (screening age, younger than screening age and older than screening age) and four time periods (1992–1996, 1997–2001, 2002–2006 and 2007–2011) among 942 breast cancer patients from Kuopio University Hospital (KUH).
The KUH as the central hospital for the North Savo Hospital District in Eastern Finland, covers a population of 248,000 in 19 municipalities, with an average of 200 new breast cancers diagnosed per year [Citation3]. By law, the nationwide, population-based Finnish Cancer Registry has registered all cancer cases in Finland since 1953. The data from the nationwide mammography screening programme have been registered since 1992 in the Mass Screening Registry, part of the Finnish Cancer Registry.
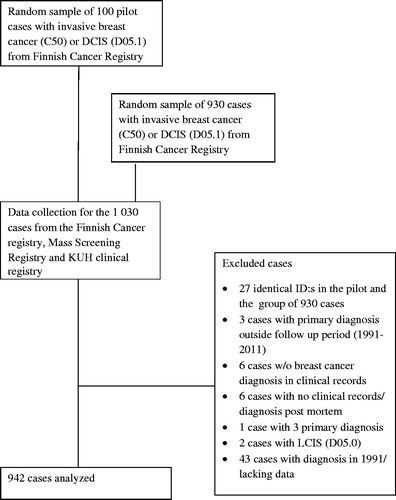
This retrospective registry study was based on 942 women diagnosed with primary breast cancer in the KUH region over a 20-year period from 1992 to 2011. Both invasive (ICD-10 code C50) and ductal in situ carcinomas (DCIS) (ICD-10 code D05.1) were included, regardless of prior malignancies. To explore the feasibility and the extent of the data collection, a pilot study with 100 patients was carried out first. The patients were randomly selected from the Finnish Cancer Registry, so that one half were diagnosed between 1991 and 1999 and the other half between 2000 and 2011. The ultimate aim was to gather 1000 patients. To achieve this, 930 more patients at the diagnostic age of 40–84 years were selected with the same method as in the pilot study. The 30 additional cases to 900 were selected because there were no in situ carcinomas in the pilot study.
There were 27 cases that were selected in both the pilot and later the main data retrieval process. In addition, from all retrieved cases, 61 were excluded because of insufficient or flawed information. These included 43 cases from 1991, the first year of initial data collection. The year 1992 was eventually selected as the first year of data collection, and thus 942 cases of first primary breast cancer were available for analyses (). If the same patient had another primary breast cancer later, only the first cancer was included in the analysis. The breast cancer was considered as bilateral if the primary breast cancer was diagnosed with less than 3-month interval in which case only the more aggressive cancer was included.
Data on diagnostic methods and tumour characteristics were collected from the clinical registry (data available since 1986) and the original patient records at KUH. Data on the method of diagnosis, location (left vs. right) and the extent of the breast cancer were gathered from the Finnish Cancer Registry database. Screening data were obtained from the National Mass Screening Registry. Screening data parameters included information on the year of invitation to screening, data from visits (screening, recalls, additional examinations) and their results.
All data were linked with the individual identification (social security) code, which is unique for every permanent citizen in Finland. The KUH clinical registry and the Finnish Cancer Registry have slightly different principles in accepting cases and only cases holding a cancer diagnosis in both registries were accepted.
To study the change in tumour characteristics over time between 1992 and 2011, the data were divided into four equal 5-year time periods: 1992–1996, 1997–2001, 2002–2006 and 2007–2011. From here forward, these are identified as the first, second, third and fourth period, respectively.
To study the changes in different ages, the cases were divided into three age groups: screening age, younger than screening age and older than screening age. Allocation to these three groups was performed by breast cancer detection mode rather than actual age due to diverse screening policies in the 19 municipalities.
The age group ‘screening age’ included women who were invited for the national screening programme and either attended, were non-participants or their invitation was not registered. Among attendees, cancers were either screen-detected or interval cancers. The upper limit of age in this group was 59–69 years depending on municipal screening policy.
The screen-detected cancers were diagnosed by the screening procedure and within 6 months from the screening invitation. Interval cancers included cancers detected between 6 and 26 months from the latest screening visit. Non-participants were women within the screening age and who were invited but did not participate during the 26 months before cancer diagnosis. The detection mode ‘not registered’ included women who should have been invited to screening according to the national screening programme but without any invitation having been registered.
The age group ‘too young’ included women who were below screening age (mostly <50 years) and their breast cancer was detected before the first screening invitation.
In the age group ‘too old’ the breast cancer was detected at least 24 months after the last screening invitation or the women were too old to be invited for screening according to the municipal screening policy (aged either 59 or 69 years).
The tumour characteristics were divided into favourable and non-favourable groups: tumours 20 mm or less in size (T1) versus larger tumours (T2–4); node-negative versus node-positive cases; ER-positive versus ER-negative tumours; grade I versus grade II–III tumours; HER2-negative versus HER2-positive tumours; and low Ki67 versus high Ki67 expression. The expression of HER2 amplification was determined by chromogenic in situ hybridization (CISH), and cancers with six or more gene copies were considered as HER2 positive. Due to the late availability of the tests for HER2, only the years 2006–2011 were included; similarly for Ki67, only the years 2007–2011 were considered. The cut-off for low versus high Ki67 was set at 15% according to the 2011 St Gallen International Expert Consensus (314; Goldhirsch et al. [Citation14]). According to the same consensus, the cut-off point for ER-positivity had been 10% until 2011.
The ER status has been determined by immunohistochemistry (IHC) during the whole study period. Frozen section samples were used in 1992–1997, and since 1998, paraffin-embedded sections were adopted for detecting the ER. Four different monoclonal antibodies have been applied over the study period (Abbot kit, DAKO Techmate 500, Novocastra and Ventana). At KUH, quality of the ER status determination is evaluated by two independent service companies focused on external quality assurance (Labquality and NordiQC) at least once annually.
The association between a tumour character and time periods was calculated using the chi-square test and age-standardized univariate analysis. Multivariate models assessed separately T class, N class, ER status, grade, HER2 status and Ki67 status with the favourable tumour character (i.e., T1, node negativity, ER positivity, grade I, HER2 negativity and low Ki67, respectively) as an index parameter. The multivariate analyses were adjusted for time period, age group and diagnostic methods (i.e., ultrasound with or without MRI and SNB for nodal status). The effect of the different age groups or diagnostic methods on the outcome variable in different time periods was assessed using interaction terms (i.e., time period × age group, time period × diagnostic method) in the multivariate models. The possible differences in the use of diagnostic methods in different age groups were tested with an interaction term age group × diagnostic method in the multivariate models. The limit for a significant interaction in a multivariate model was set to p < .05.
In situ carcinomas and cases with unknown tumour characteristics are presented in the baseline characteristics table but were excluded from the final analyses. The detection mode ‘not registered’ was excluded from the final analysis due to low number of cases (n = 13). Statistical analyses were performed with IBM SPSS version 22 for Windows (SPSS Inc., Chicago, IL, USA).
Permission for this study was provided by the National Institute for Health and Welfare (THL/457/5.05.00/2012) and the Ethics Committee of the University of Eastern Finland. The research subjects were not contacted.
Results
Study population
The study included 942 patients, of whom 457 were diagnosed in 1992–1999 and 485 in 2000–2011. The tumour characteristics by time periods are presented in .
Table 1. Patient and tumour characteristics.
The mean age at the time of diagnosis increased from 60.3 (34–86) years in the first period to 63.1 (38–87) years in the fourth period. Accordingly, the proportion of ‘too young’ decreased from 25.6% to 11.1% and the proportion of ‘screening age’ increased from 19.4% to 32.2%. Ductal histology was most common at 67.0%. The proportion of lobular histology was low, at 8.8% in the first period but increasing thereafter and comprising 15.2% of the whole population. The proportion of DCIS did not change.
The proportion of unknown tumour characteristics was greatest in the first period, but still in the fourth period 10.2% of the cases with invasive breast cancer had an unknown grade status and 5.9% had an unknown ER/PgR status.
Implementation of diagnostic methods
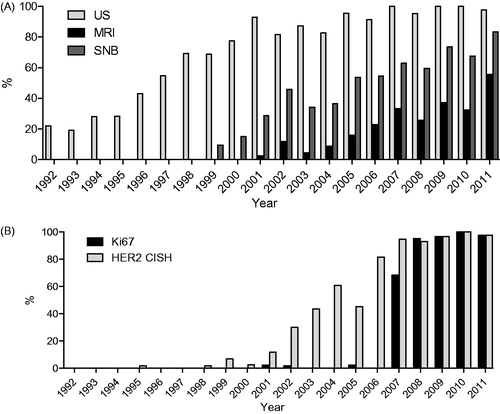
Employment of new diagnostic methods increased rapidly after their introduction (). The use of ultrasound in BC diagnostics became more common over time, as it was used in 22.0% of cases in the first period but in practically 100% in the fourth period. The MRI was first employed in 2001 when it was used in 2.3% of cases, while by 2011 it was used in almost half of the cases. Ki67 evaluation was very rapidly adopted after 2007 and was performed in nearly every case in 2011. HER2 status was first examined in some occasional cases with metastatic disease by immunohistochemistry (IHC) staining in 1996. The CISH of HER2 was introduced in 2006–2007 and has been performed in nearly all invasive cases since 2007. Intraoperative SNB was introduced in 1999 and was used in 80% of cases in 2010.
The change of tumour characteristics
ER
There was a clear secular increase in ER-positive cancers. The proportion of ER-positive cancer increased from 65.1% in 1992–1996 to 84.1% in 2007–2011 (). In the age-standardized analysis, the shift was significant in every consecutive time period when compared to the first period; odds ratios and confidence intervals for the second, third and fourth period, respectively, were OR 2.0; 95% CI 1.27–3.17, OR 2.0; 95% CI 1.23–3.24, and OR 2.71; 95% CI 1.61–4.55.
Table 2. Tumour characteristics of invasive breast cancers by time periods.
The second (OR 1.82; 95% CI 1.10–3.01) and the fourth (OR 1.95; 95% CI 1.01–3.80) periods also remained significantly associated with ER positivity in the multivariate analysis. Somewhat more ER positivity was seen among ‘too old’ (OR 1.48; 95% CI 0.99–2.22) when compared to ‘screening age’, but the effect of the age groups on ER positivity did not differ between the time periods (interaction p = .99), that is, the secular change was detected in all age groups. The use of ultrasound or MRI did not predict ER positivity in any time period ().
Table 3. Relative risk of T1, N−, ER + and grade I cancers adjusted for time periods, age groups and different diagnostic methods.
T-stage
There was no clear secular trend in the proportion of T1 cancers (). However, there was a statistically significant increase in T1 cancers in the age-standardized analysis in the fourth period when it was compared to the third period (OR 1.75; 95% CI 1.16–2.65).
In the multivariate analysis of all time periods, significantly more T1 cancers were seen among women at ‘screening age’ than among ‘too young’ (OR 0.63; 95% CI 0.43–0.94) or ‘too old’ (OR 0.60; 95% CI 0.44–0.83) ().
In a separate multivariate analysis restricted to the third and fourth periods, the fourth period remained as an independent predictor of T1 cancer (OR 1.68; 95% CI 1.08–2.62). Also, in the third and fourth period, cancers of women at ‘screening age’ were more often T1 cancers than the cancers of ‘too young’ (OR 0.44; 95% CI 0.22–0.86) or ‘too old’ (OR 0.64; 95% CI 0.40–1.02).
The influence of screening age on T-stage was detected despite the time periods (interactions p = .84 for all time periods and p = .83 for the third and fourth periods). The use of ultrasound or MRI did not predict T-stage in any time period ().
N-stage
There was no clear secular trend in the proportion of node-negative cancers. However, there was a statistically significant increase in node-negative cancers in the age-standardized analysis when the fourth period was compared to the third period (OR 1.58; 95% CI 1.04–2.39) ().
The use of SNB as a diagnostic method increased () since the second period. Thus, the effect of SNB on the risk of node negativity was significantly dependent on time period (interaction p = .023) and the time periods were therefore separately analysed.
In the multivariate analysis, SNB was an independent predictor of node negativity in the third (OR 4.16; 95% CI 2.13–8.09) and fourth (OR 9.08; 95% CI 4.42–8.64) periods. In the multivariate analysis, if either ultrasound or both ultrasound and MRI were performed, the risk of node negativity was significantly reduced when compared to the situation where neither of these methods was applied in the third period (OR 0.22; 95% CI 0.06–0.88 and OR 0.17; 95% CI 0.03–0.84, respectively) ().
In a separate multivariate analysis restricted to the third and fourth periods, the increase in node-negative cancers in the fourth period was not statistically significant. But also in this analysis, the use of SNB significantly predicted node negativity (OR 6.05; 95% CI 3.72–9.83), and the use of ultrasound or both ultrasound and MRI reduced the risk of node negativity (OR 0.20; 95% CI 0.05–0.80 and OR 0.17; 95% CI 0.04–0.75, respectively).
Grade
There was no secular change in the risk of grade I cancers in the age-standardized analysis or in the multivariate analysis. Age group, the use of ultrasound or both ultrasound and MRI were not associated with the risk of grade I cancer ().
HER2
Since 2006, 214 cases of invasive breast cancer were included in the study and 19.2% (n = 40) of them were HER2 positive. In the multivariate analysis of the age group, the use of ultrasound or use of both ultrasound and MRI was not associated with HER2 status at diagnosis.
Ki67
Since 2007, Ki67 was assessed in 91.4% cases (171/187), of which 41.5% (n = 71) had a low Ki67. In the multivariate analysis, neither the age group nor the diagnostic methods were associated with Ki67 status.
Discussion
We explored the change of breast cancer characteristics and their association with the parallel introduction of diagnostic methods in three age groups (screening age, beyond screening age and under screening age) over four time periods (1992–1996, 1997–2001, 2002–2006 and 2007–2011). According to the present study, the most discernible secular change was the increase in ER-positive cancers in every consecutive time period. The risk of ER positivity in the second, third and fourth periods was 2- to 2.71-fold as compared to the first period. The change could not be explained by the employment of new diagnostic methods. An increase in small tumours and node-negative tumours was detected during the most recent years of data collection. The secular changes were detected in all age groups, but overall ER positivity was most frequent among women beyond screening age and small tumours were most frequent among screening-aged women.
In developed countries, the risk of ER-positive breast cancer has been associated with reproductive factors and the use of combined oral contraceptives or hormone replacement therapy (HRT) [Citation18–20]. In our study, the secular change was not dependent on age group, but overall ER positivity was most frequent among the ‘too old’ group, that is, the age group with potentially the longest exposure to both exogenous and endogenous ER.
In a US SEER register-based study, the proportion of ER-positive cancers increased along with age. The age-standardized incidence of ER-positive cancer was highest among women 50–84 years of age but increased especially among young women 30–49 years of age during 1992–2008. However, among women 50–84 years of age, the incidence of ER-positive cancers increased only until the year 2000 and then levelled back to that of the early 1990s [Citation21], reflecting perhaps the use of HRT. In a similar Danish study, the rate of ER-positive cancer was highest among postmenopausal women and increased with age especially after age 50 years [Citation22]. Both of these studies estimated the annual percentage change of ER-positive cancer (0.75% per year and 3.0% per year, respectively) and considered increasing ER positivity as a future trend.
In the present study, the increase in ER-positive cancers in the first study years can be partly explained by methodological changes in handling the tumour samples: a delay in fixation of the sample may have caused an error (ER negativity), which was corrected in the later years. The technology of the equipment in the pathology laboratory has also improved over time. Despite the above-mentioned methodological problems, the increase in ER-positive cancer was most obvious over the last time period. The secular increase in ER positivity rate was detected in all age groups and thus there might be many different explanatory factors. The use of HRT and hormonal contraceptives has changed during the decades in addition to changes in dietary factors, alcohol intake and physical activity. In other studies, increased alcohol intake and physical inactivity have been associated especially with the expression of ER-positive breast cancer [Citation23–25]. Obesity and high body mass index (BMI) might be important explaining factors, because in addition to hormonal mechanisms obesity is associated with chronic inflammation [Citation26,Citation27]. Interestingly, in this study the proportion of lobular histology almost doubled during the time period, supporting the role of hormonal factors, because the use of HRT is associated especially with lobular subtype [Citation28,Citation29].
During the most recent years, there has been an increase in small tumours and node-negative tumours. In two recent studies, an increasing proportion of small tumours and node-negative tumours were observed after the introduction or extension of population-based screening [Citation30,Citation31]. In Finland, the extension of population-based mammographic screening from women of 50–59 years to 50–69 years was not concurrent in all municipalities, but the majority of the municipalities in the KUH district implemented the extension in 2007. This could partly explain the increased proportion of T1 cancers and node-negative cancers in the fourth period. On the other hand, the advances in imaging methods (e.g., the digitalization of mammography) may have attributed to increased detection of T1 tumours. In the KUH region, the screening mammography was digitalized in March 2006, that is, just before the last time period. In agreement, in a recent Spanish study, the shift from screen-film mammography to full-field digital mammography also resulted in an increase in small invasive cancers (<20 mm) [Citation32].
In the present study, intraoperative SNB was a significant predictor of node negativity. The association was anticipated since SNB is performed only if there is no evidence of node positivity in the preoperative imaging studies, while patients with known lymph node positivity proceed straight to axillary lymph node dissection. There was an inverse association between node negativity and the use of ultrasound or both ultrasound and MRI in the third period, that is, more node-positive cancers were detected if these imaging methods were applied. In the third period, the use of ultrasound was still increasing and the use of MRI was introduced, which may have improved the detection and localization of lymph nodes and thus improved the accuracy of lymph node dissection and increased node positivity. In the fourth period, ultrasound was performed to practically all cases and therefore no association between preoperative imaging and lymph node status could be detected.
The proportion of HER2-positive cancers (19.2%) was relatively high, while in previous studies the respective proportion has been 14.7–16.0% [Citation33]. In the current study, HER2 status was detected by CISH, that is, by a highly sensitive method [Citation34], and thus the false-negative rate should be low. According to our study, age does not influence HER2 status, which is interesting as the aetiology of HER2 positivity is unknown.
This study aimed to explore the change of breast cancer characteristics in parallel to the implementation of novel diagnostic methods. Previous studies covering the topic have focused mainly on a single technique, while in this study all modern techniques were included. Advanced diagnostic methods have decreased the proportion of tumours with unknown characteristics and improved the detection of breast cancer in its early phase. This study suggests that small tumours are detected especially at screening age and ER-positive tumours among women beyond screening age. However, the secular changes are seen over all ages.
Considering the widely adopted western lifestyle associated with physical inactivity, obesity and increased alcohol intake, the trend of increasing ER positivity is very likely to continue in the future as also estimated in the aforementioned US and Danish studies. When considering modern sensitive imaging methods, it seems that we are going to have a breast cancer population of mainly small ER-positive breast cancers in the future. The change of tumour characteristics may influence the effectiveness of current treatment options and force an alteration of the therapeutic approach.
Acknowledgments
The authors acknowledge biostatistician Tuomas Selander for his assistance in data analysis and M.D. Eero Kumpulainen for his assistance in data collection.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Altobelli E, Lattanzi A. Breast cancer in European Union: an update of screening programmes as of March 2014 (review). Int J Oncol. 2014;45:1785–1792.
- Hakama M, Pukkala E, Heikkila M, et al. Effectiveness of the public health policy for breast cancer screening in Finland: population based cohort study. Br Med J. 1997;314:864–867.
- Finnish Cancer Registry. [cited 2018 Feb]. Available from: https://www.Cancer.fi/syoparekisteri/
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998–2005.
- Lousdal ML, Kristiansen IS, Moller B, et al. Effect of organised mammography screening on stage-specific incidence in Norway: population study. Br J Cancer. 2016;114:590–596.
- Levi F, Bosetti C, Lucchini F, et al. Monitoring the decrease in breast cancer mortality in Europe. Eur J Cancer Prev. 2005;14:497–502.
- Carioli G, Malvezzi M, Rodriguez T, et al. Trends and predictions to 2020 in breast cancer mortality in Europe. Breast. 2017;36:89–95.
- Ohuchi N, Suzuki A, Sobue T, et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan strategic anti-cancer randomized trial (J-START): a randomised controlled trial. Lancet. 2016;387:341–348.
- Nothacker M, Duda V, Hahn M, et al. Early detection of breast cancer: benefits and risks of supplemental breast ultrasound in asymptomatic women with mammographically dense breast tissue. A systematic review. BMC Cancer. 2009;9:335–2407.
- Skaane P. Studies comparing screen-film mammography and full-field digital mammography in breast cancer screening: updated review. Acta Radiol. 2009;50:3–14.
- Kerlikowske K, Hubbard RA, Miglioretti DL, et al. Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: a cohort study. Ann Intern Med. 2011;155:493–502.
- Sardanelli F, Boetes C, Borisch B, et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer. 2010;46:1296–1316.
- Heikkinen S, Miettinen J, Koskenvuo M, et al. Proportion of women with self-reported opportunistic mammography before organized screening. Acta Oncol. 2016;55:865–869.
- Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747.
- Crispo A, Barba M, D’Aiuto G, et al. Molecular profiles of screen detected vs. symptomatic breast cancer and their impact on survival: results from a clinical series. BMC Cancer. 2013;13:15.
- Hofvind S, Holen A, Roman M, et al. Mode of detection: an independent prognostic factor for women with breast cancer. J Med Screen. 2016;23:89–97.
- Redondo M, Funez R, Medina-Cano F, et al. Detection methods predict differences in biology and survival in breast cancer patients. BMC Cancer. 2012;12:604–2407.
- Anderson KN, Schwab RB, Martinez ME. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res Treat. 2014;144:1–10.
- Collins JA, Blake JM, Crosignani PG. Breast cancer risk with postmenopausal hormonal treatment. Hum Reprod Update. 2005;11:545–560.
- Beral V. Million women study collaborators. Breast cancer and hormone-replacement therapy in the million women study. Lancet. 2003;362:419–427.
- Anderson WF, Katki HA, Rosenberg PS. Incidence of breast cancer in the United States: current and future trends. J Natl Cancer Inst. 2011;103:1397–1402.
- Anderson WF, Rosenberg PS, Petito L, et al. Divergent estrogen receptor-positive and -negative breast cancer trends and etiologic heterogeneity in Denmark. Int J Cancer. 2013;133:2201–2206.
- Kerr J, Anderson C, Lippman SM. Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. Lancet Oncol. 2017;18:457–471.
- Lope V, Martin M, Castello A, et al. Physical Activity and breast cancer risk by pathological subtype. Gynecol Oncol. 2017;144:577–585.
- Li CI, Chlebowski RT, Freiberg M, et al. Alcohol consumption and risk of postmenopausal breast cancer by subtype: the women's health initiative observational study. J Natl Cancer Inst. 2010;102:1422–1431.
- Crespi E, Bottai G, Santarpia L. Role of inflammation in obesity-related breast cancer. Curr Opin Pharmacol. 2016;31:114–122.
- Reggiani F, Bertolini F. Roles of obesity in the development and progression of breast cancer. Discov Med. 2017;24:183–190.
- Ravdin PM. Hormone replacement therapy and the increase in the incidence of invasive lobular cancer. Breast Dis. 2008;30:3–8.
- Dossus L, Benusiglio PR. Lobular breast cancer: incidence and genetic and non-genetic risk factors. Breast Cancer Res. 2015;17:37.
- Berube S, Provencher L, Robert J, et al. Quantitative exploration of possible reasons for the recent improvement in breast cancer survival. Breast Cancer Res Treat. 2007;106:419–431.
- Jensen MB, Ejlertsen B, Mouridsen HT, et al. Improvements in breast cancer survival between 1995 and 2012 in Denmark: the importance of earlier diagnosis and adjuvant treatment. Acta Oncol. 2016;55:24–35.
- Sala M, Domingo L, Macia F, et al. Does digital mammography suppose an advance in early diagnosis? Trends in performance indicators 6 years after digitalization. Eur Radiol. 2015;25:850–859.
- Pathmanathan N, Provan PJ, Mahajan H, et al. Characteristics of HER2-positive breast cancer diagnosed following the introduction of universal HER2 testing. Breast. 2012;21:724–729.
- Zhao J, Wu R, Au A, et al. Determination of HER2 gene amplification by chromogenic in situ hybridization (CISH) in archival breast carcinoma. Mod Pathol. 2002;15:657–665.