Abstract
Introduction: Increased knowledge of breast cancer risk factors may enable a paradigm shift from the current age-based mammographic screening programmes to a personalised risk-based approach. This would warrant a significant change in practice, yet the acceptability from a woman’s perspective has never been systematically explored. In this systematic review, we inventoried and appraised studies of women’s perceptions of risk-based breast cancer screening and prevention to identify factors associated with adopting this new paradigm.
Methods: We searched Medline, Embase and PsycInfo to identify original articles in English containing perceptions of risk-based breast cancer screening and/or primary prevention of women with an average to above average risk of developing breast cancer. Qualitative data were systematically extracted and referenced against four theoretical models of preventative health behaviour adoption.
Results: When considering the adoption of this novel screening and prevention programme, women carefully review their perceived susceptibility to breast cancer. Their decisions are based on a cost–benefit analysis of adopting lifestyle changes, chemoprevention, or prophylactic surgery, taking into account their perceived competence, individual autonomy, relatedness to others, and personal preference. The role of intent is limited when considering behavioural change.
Conclusions: Implementing risk-based breast cancer screening and prevention will require a multifactorial approach. The transition from theory to practice can be supported by developing evidence-based shared decision aids and family-oriented (genetic) counselling programmes.
Introduction
Increased knowledge of breast cancer risk factors may enable us to move from current ‘one-size-fits-all’ population-based breast cancer screening programmes to a more personalised risk-based approach. A woman’s screening strategy could be optimised by integrating individual variations in breast cancer risk [Citation1]. The efficiency of risk-based breast cancer screening has been modelled showing increased detection of breast cancers in younger women at higher risk, a potential reduction in false-positive outcomes and overdiagnosis, and improved cost-effectiveness [Citation2]. Hence, this approach could improve the balance between the benefits and harms of screening for women with either a lower or higher than average risk of developing breast cancer [Citation2]. Classifying women according to breast cancer risk at a population level will also provide greater opportunities for primary prevention. Women can attempt to actively reduce their breast cancer risk by participating in a lifestyle programme or taking risk-reducing medication (chemoprevention). Risk-based breast cancer screening and prevention would require extensive changes to current practice and women’s participation is a prerequisite for potential cost-effectiveness and successful implementation [Citation3]. Yet, the acceptability of risk-based breast cancer screening and primary prevention has never been systematically explored from the perspective of eligible women.
Currently, prevention strategies, such as risk-reducing medication, are only discussed with women at high risk of developing breast cancer (i.e., 10-year risk ≥8%) [Citation4]. However, the National Institute of Health and Care Excellence (NICE) guidance states that risk-reducing medication should also be mentioned to women with a moderate risk of breast cancer (i.e., 10-year risk of 5–8%) [Citation4]. In addition, all women, regardless of breast cancer risk, may benefit from information on healthy lifestyle behaviours, especially as behaviours like a high calorie intake diet and lack of exercise are also implicated in other medical conditions [Citation5].
The introduction of personalised risk-based screening and subsequent primary prevention would require women to make substantial changes to their current screening and preventative health behaviours. Exploring factors that may influence women’s adoption of the novel programme will provide valuable insights for future implementation. Therefore, the objective of the present study is to systematically explore and appraise studies of women’s perceptions of integrated risk-based breast cancer screening and prevention. Based on this analysis, we will develop a conceptual framework illustrating the factors that potentially influence women’s acceptability of this new screening and prevention programme for breast cancer.
Methods
Search strategies
We used two search strategies to identify articles on women’s perceptions of the adoption of personalised risk-based breast cancer screening and prevention. The construct ‘perceptions’ was operationalised to include ‘attitudes’ and ‘perspectives’ to achieve a broad overview of women’s reactions to the new screening strategy. Medline (1966–January 2016), EMbase (1974–January 2016) and PsycINFO (1806–January 2016) were searched to obtain a comprehensive result. The complete search strategies are available in Supplement 1. Reference lists of relevant reviews and the included articles were screened to identify additional studies.
Selection criteria
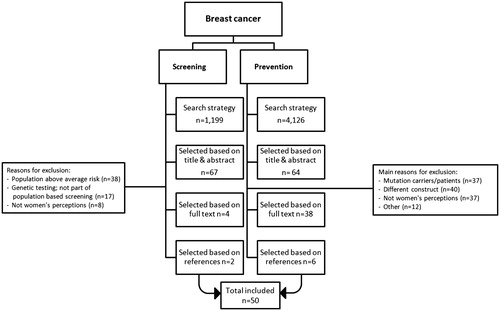
An article was selected for inclusion when it concerned a full-text original article in English (i.e., not a review), aiming to evaluate perceptions of personalised risk-based breast cancer screening (of women with an average risk of developing breast cancer) and/or primary prevention (of women with an average to above average risk of developing breast cancer). This distinction was made because women who attend screening are considered to have a population-average risk of developing breast cancer, whereas prevention is generally aimed at women who have an increased risk of developing breast cancer. Exclusion criteria were: study population of BRCA1/2 carriers or women who had previously been diagnosed with breast cancer, perspectives of (healthcare) professionals, perceptions of current population-based screening programmes, and articles which solely evaluated the effectiveness of an intervention (not considering women’s perceptions). Two researchers independently screened each title, abstract, and full-text article (LR, DvdW, AJ, YW, MB). One researcher appraised the methodological quality of the included studies (LR). When disparities arose, consensus was reached through discussion.
Methodological quality assessment
Studies were appraised using the Critical Appraisal Skills Programme (CASP) tool from the National Health Service Public Health Resource unit [Citation6]. This tool allows a systematic inventory of the quality and validity of studies, is easily accessible, and is commonly used in systematic reviews. Subsequently, relevant data were collected using a data extraction form containing information on the study population, recruitment methods, design, main outcomes, and generalisability. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline was used to guide the reporting of this systematic review [Citation7].
Synthesis of results
Although we intended to perform a quantitative meta-analysis, the included studies did not meet the established requirements [Citation8]. Since ‘acceptability’ is a relatively broad term, it was defined and measured differently in the included studies, preventing meaningful insights from averaging the results. To summarise the quantitative research, the key quantitative findings are presented systematically (Supplement 2). In this review, we focus on the qualitative results regarding the main outcome defined as women’s perceptions of risk-based screening and preventative behaviour adoption.
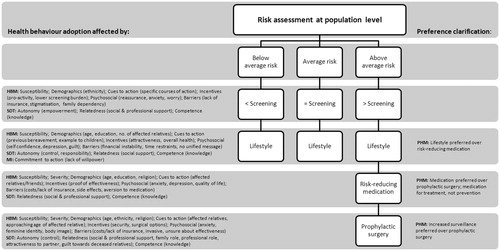
The qualitative data were systematically extracted from each study and analysed without any preconceived theories in mind using Braun and Clarke’s method, i.e., familiarisation with the data, organising data into meaningful groups using a data-driven strategy, developing themes by evaluating overarching topics and relationships, and studying the interconnectedness of the topics [Citation9]. This leads to the identification of meaningful qualitative statements and themes. These extracted statements and themes were subsequently linked to four known theoretical models which aim to explain breast cancer health behaviour adoption, i.e., motivation interviewing (MI), the health belief model (HBM), self-determination theory (SDT) and the preventive health model (PHM) [Citation10]. provides an overview of the four theoretical models. In the analysis, we distinguished between women’s perceptions of risk-based screening, lifestyle changes, chemoprevention, and prophylactic surgery.
Table 1. Overview of the four theoretical models and the definitions of their underlying constructs.
Results
Characteristics of included studies
The search strategy for women’s perceptions of risk-based breast cancer screening identified 1199 unique publications, of which we selected 67 articles based on title and abstract. Full-text screening resulted in the selection of four articles. Reference checking identified two additional studies. In total, six articles on risk-based screening were included (). The search strategy for women’s perceptions of breast cancer prevention identified 4126 unique publications; 164 articles were selected after title and abstract screening. Full-text screening resulted in the selection of 38 articles. Reference checking identified six additional eligible studies, leading to a total of 50 studies on risk-based screening and prevention. All included studies were appraised to be of average to good quality [Citation6]. Supplement 2 gives an overview of the general characteristics and the key quantitative outcomes of the included studies. Supplement 3 provides detailed qualitative information of women’s perceptions of risk-based breast cancer screening and prevention. Lastly, Supplement 4 provides an overview of the extent to which the four theoretical models were represented in the articles.
Women’s perceptions of personalised risk-based screening and prevention
shows the theoretical framework for risk-based breast cancer screening and preventative health behaviour adoption from the perspective of women. It illustrates how women’s perceptions of this novel programme relate to the four theoretical models of health behaviour adoption. The HBM and SDT were applicable to women’s perceptions of risk-based screening and all identified methods of prevention, i.e., lifestyle (n = 28 studies), chemoprevention (n = 8), and prophylactic surgery (n = 8). The theoretical model of MI was only applicable to women’s perceptions of preventative lifestyle changes. Women’s perceptions of all preventative practices could be linked to PHM, although PHM was not applicable to their perceptions of risk-based screening. We describe women’s perceptions of risk-based breast cancer screening and prevention in more detail below, relating it to the four models and their key constructs (in italics).
Health belief model
Women reported favourable opinions about receiving risk information to tailor breast cancer screening and prevention [Citation11–13]. Perceived susceptibility influenced perceptions of risk-based screening and prevention, as higher perceived risk was associated with a higher acceptance of increased screening frequency, lifestyle changes, chemoprevention, and surgery [Citation14–26]. Low perceived risk, however, did not reduce perceived susceptibility, limiting the endorsement of a lower screening frequency [Citation12,Citation14]. Additionally, perceived health influenced women’s acceptability of chemoprevention, with healthier women feeling less susceptible to breast cancer and therefore less interested in chemoprevention [Citation27,Citation28]. Women’s perceived severity of breast cancer was affected by having relatives who had been diagnosed with breast cancer and by a more acute perception of other medical conditions, e.g., diabetes or cardiovascular disease. The former made women more likely to accept prophylactic surgery, the latter made women less likely to accept chemoprevention [Citation21,Citation27,Citation29–32]. Several incentives for personalised risk-based screening were identified, such as the feeling of empowerment through knowledge and the possibility to screen more efficiently, reducing the screening burden for low risk women [Citation11,Citation13]. Looking more attractive, reducing menopausal symptoms, and reducing the risk of other medical conditions were identified as incentives for the adoption of lifestyle changes [Citation33,Citation34]. The main incentive to chemoprevention acceptance was comprehensive information on the effectiveness of chemoprevention [Citation17,Citation27,Citation35]. Surgical options after mastectomy, e.g., breast augmentation were incentives to opt for prophylactic surgery [Citation24,Citation29,Citation36]. Barriers to risk-based screening included the need for family risk communication, a lack of insurance coverage, and possible employer/insurance discrimination [Citation11–13,Citation37]. Barriers to the acceptance of lifestyle changes were costs, time investment, and the lack of a clear message on how lifestyle affects breast cancer risk [Citation15,Citation33]. A main barrier to chemoprevention was the perception of potential side effects, with women consistently overestimating the risk of developing complications after chemoprevention use [Citation18,Citation19,Citation27,Citation28,Citation31,Citation32,Citation38–46]. Main barriers to prophylactic surgery included not having health insurance or financial stability, the perceived radical nature of the procedure, and relying on prayer and spirituality [Citation24,Citation47]. Feeling happier and overcoming depression were psychosocial factors associated with higher acceptance of healthy lifestyle changes [Citation33]. Increased cancer worry, anxiety and intrusive thinking were associated with higher interest in chemoprevention and prophylactic surgery (psychosocial) [Citation18,Citation19,Citation21,Citation22,Citation24,Citation30,Citation48–50]. However, the perception that losing your breasts would affect body image and identity limited acceptance of prophylactic surgery (psychosocial) [Citation24,Citation29,Citation51].
Self-determination theory
Women reported feeling insecure about the potential impact personal risk information could have on their lives and their ability to manage any potential consequences (competence) [Citation11]. A perceived lack of knowledge limited acceptance of risk-based screening and all preventative methods [Citation20,Citation36,Citation44,Citation47,Citation51]. Lacking faith in own ability (competence) hindered participation in a lifestyle programme specifically [Citation33,Citation47]. Moreover, women indicated that having to decide on chemoprevention was a great responsibility (competence), worrying about disrupting their current state of health [Citation28,Citation32,Citation38]. The term ‘chemoprevention’ elicited especially strong reactions from women [Citation17,Citation31]. Perceiving control over and accepting responsibility for own health (autonomy) were motivators for adopting preventative behaviours [Citation17,Citation32,Citation39]. Additionally, the importance of social support from friends and family (relatedness) was emphasised in the decision-making process for risk-based screening and prevention in general [Citation20,Citation30,Citation31,Citation33,Citation36,Citation38,Citation44,Citation47,Citation50,Citation52–54].
Motivation interviewing
Women generally accepted lifestyle changes for reducing breast cancer risk, however, they were concerned that they lacked the willpower to affect change (commitment to action) [Citation15,Citation33,Citation47,Citation55]. One study confirmed this, showing high intent but low behavioural change (e.g., diet and exercise) after breast cancer risk communication [Citation33].
Preventive health model
Several studies compared women’s perceptions of chemoprevention and prophylactic surgery. Although women generally preferred chemoprevention to surgery, they required a guaranteed risk reduction of 50–100% to conceive of either procedure as acceptable preventative options (preference clarification) [Citation17,Citation50,Citation56,Citation57]. Moreover, increased surveillance was preferred to prophylactic surgery (preference clarification) [Citation30,Citation50].
Women’s communication and information needs regarding personalised risk-based screening and prevention
Women identified the screening organisation as being the most suitable setting to perform a risk assessment, prior to mammography screening [Citation11]. However, women were divided about their preferred way of being informed about their risk of breast cancer. Some preferred a consultation, a telephone call, or an e-mail, either from a cancer specialist or a GP [Citation12]. Women who were offered the option of receiving personalised breast cancer risk information on a secure website responded positively [Citation58]. Additionally, women would welcome supplementary printed materials, interactive group education sessions, and support groups to help them cope with personal risk information [Citation58].
The primary care physician was identified as the most important source of information on chemoprevention [Citation42]. Women’s information needs included the reliability and effectiveness of medication, and decision-making guidance [Citation40,Citation59]. Women prefer the physician to assume a directive role, taking a woman’s personal preference into account [Citation40]. Women have difficulty differentiating between a physician lacking knowledge and knowledge not being available [Citation60]. Physicians expressing uncertainty can either be perceived as anxiety inducing or as truthful [Citation60]. Women suggested several options for improving physician–patient communication, e.g., additional training to educate physicians on the importance of shared informed decision-making, getting a second opinion, developing culturally appropriate intervention projects, and creating an open environment where both appreciation and grievances can be expressed [Citation42]. Most women felt that some form of counselling or a support group was needed after risk communication, because they felt isolated [Citation30,Citation40]. Communicating decisions on prevention to family members was perceived as difficult, with women experiencing relatives’ fear, shock and bereavement [Citation29,Citation30,Citation51].
Discussion
This review provides a framework for the factors that may influence women’s participation in personalised risk-based breast cancer screening and prevention (). Decision-making about the adoption of this programme appears best described by a cognitions-based process, whereby women evaluate the perceived threat of breast cancer and consider the pros and cons of available preventative strategies in their social context. The limited role of intent is unsurprising and in line with previous research, which shows that interventions relying solely on intent had limited effects on health behaviour change [Citation61]. Seeking behaviour change through intent is particularly challenging for those women who perceive a low sense of control over their own health [Citation62]. Moreover, breast cancer prevention requires relatively ambiguous and long-term efforts.
Adopting risk-based screening
Women appear in favour of receiving personalised information on breast cancer risk, with all studies showing a generally positive attitude. Women’s general interest in breast cancer risk information has previously been demonstrated [Citation63]. Evans and colleagues recruited women from the United Kingdom National Health Service breast screening programme and found that 95% of their study participants wanted to know their personal breast cancer risk. Additionally, receiving risk information had no adverse effect on screening attendance for high risk women who were more likely to attend their subsequent mammogram [Citation64]. Our review showed that incentives for women’s uptake of a risk-based screening programme include increased knowledge, a sense of empowerment, and the inclusion of lifestyle factors to increase perceived control over breast cancer risk. This confirms that receiving exclusively genetic risk information limits women’s motivation and hinders potential behavioural change [Citation65]. The prominent role of perceived control in health behaviour adoption has previously been demonstrated in a review of women carrying a BRCA mutation [Citation66].
Adopting lifestyle changes
Overall, women appeared to welcome the opportunity to modify their personal risk through diet and exercise with four of the seven studies on lifestyle changes reflecting an accepting attitude. Women’s apparent acceptance is a potentially important finding, as it is feasible that the Western lifestyle – characterised by dietary and reproductive changes, and increased sedentary activity – has contributed to the worldwide increase of breast cancer cases [Citation63]. Adopting a healthy lifestyle, in particular sustained postmenopausal weight reduction, could lead to a breast cancer risk reduction of around 25% [Citation67]. However, a woman’s intention to improve lifestyle does not guarantee behavioural change [Citation60,Citation68]. In this review, we show that women reported a general lack of commitment to lifestyle changes because they believe they lack the willpower. A prominent factor hindering the uptake of lifestyle changes was the absence of a clear message on the relation between lifestyle and breast cancer risk. Primary care facilities or screening providers could be opportune places to increase women’s knowledge by providing group education sessions collectively endorsing national guidelines on cancer prevention lifestyle practices [Citation69]. This could improve general feelings of competence and autonomy, as well as improving the perceived cost–benefit balance of adopting healthy risk-reducing lifestyle behaviours.
Adopting prophylactic surgery and chemoprevention
The consideration of prophylactic surgery and chemoprevention in the general population was contingent on a (very) high perceived breast cancer risk (75–100%). The fact that prophylactic surgery lacks general acceptance is unremarkable, as this procedure was discussed with women who do not meet the risk requirements. Women eligible for prophylactic surgery are usually identified as high risk prior to screening and are therefore generally already under surveillance at a hospital or a health clinic [Citation4].
Women’s apparent limited acceptance of chemoprevention is more relevant, because more women are expected to meet the eligibility criteria after breast cancer risk assessment. Studies performed at screening centres and general practices identified an additional 3% of women meeting the Tyrer–Cuzick cut-off criterion for moderate risk and thus chemoprevention [Citation63,Citation70,Citation71]. These women would previously have gone undetected by only inventorying family history. Clinical trials have demonstrated breast cancer risk reductions of 35–40% [Citation71]. The general uptake of and adherence to chemoprevention is low, despite its proven effectiveness in clinical trials and its endorsement by the Food and Drug Administration, the American Society for Clinical Oncology and the National Comprehensive Cancer Network [Citation72]. Acceptance of chemoprevention in our study appears to be mainly hindered by the severity of the perceived side effects. Beneficial effects of lower tamoxifen doses on breast cancer incidence have been observed [Citation73]. Therefore, determining the minimal effective dose could improve uptake. Additionally, two studies reported a general aversion to the name chemoprevention, as it elicited painful memories and associations with chemotherapy. Several alternative names have been suggested, e.g., preventative therapy or risk-reducing medication [Citation74]. These names may elicit milder responses, leaving more room for deliberation.
Risk communication
Women consistently identified the opinion of the physician as a motivator to adopt risk-based screening and prevention. This demonstrates the importance of optimal communication with care-providers. The significant challenges associated with health communication in general and risk communication in particular have previously been evaluated in a comprehensive review [Citation75]. This review showed that women who were presented with a personal breast cancer risk often misunderstood the (long-term) implications of their risk. Understanding risk information is a vital step in the adoption of tailored preventative behaviours. Previous recommendations for improving the quality of risk communication in practice are in line with the information needs of women identified in our review, e.g., preference for printed materials and web-based risk communication formats [Citation76]. This highlights the relevance of developing evidence-based, shared decision-making protocols to enable women to increase their perceived autonomy, competence and sense of relatedness, aiding potential preventative health behaviour adoption.
In addition, women emphasised their relatedness to significant others, e.g., friends, relatives, partner and children. To date, risk-related communication in the family setting has received little attention, but this is an essential part of this new screening and prevention programme. This review shows that women feel socially isolated, are concerned for family members’ reactions, fearful of judgement, and worried about reiterating past bereavement regarding relatives’ missed prevention opportunities, breast cancer diagnosis, and breast cancer related deaths. These family-oriented themes were also identified in a study among women who were at high risk of carrying a BRCA mutation [Citation77]. Discussions with potential BRCA carriers established five family-related themes, i.e., distress about possible transmission to children, family conflict about testing, concerns about disclosure, different coping styles and decision-making, and underlying family conflict and unresolved grief [Citation77]. This highlights the need to counsel women on how to communicate risk information and subsequent preventative choices to family members to aid potential health behaviour adoption.
Strengths and limitations
To our knowledge, this review proposes the first framework of women’s perceptions of a novel risk-based breast cancer screening and subsequent prevention programme. Although effects of breast cancer risk feedback, general decision-making, and uptake of prevention have previously been studied [Citation75,Citation78], there is a lack of information on women’s motivations behind potential adoption or rejection of the integrated early detection and prevention programme. However, our proposed framework has one main limitation, namely that women’s perceptions of risk-based screening and prevention were generally explored outside of the infrastructure of a population-based screening programme. Unfortunately, studies in which this new screening and prevention paradigm is already embedded in current practice are rare, yet this is pivotal for future implementation.
The comprehensive and systematic nature of the literature search allowed for an extensive overview of women’s perceptions of personalised risk-based screening and primary prevention for breast cancer. Although the search identified both quantitative and qualitative study results, a meta-analysis of quantitative data was not attainable, due to the heterogeneity in the measurement of study determinants and outcomes. To increase the comparability of future studies, consensus is required on the aspects which define acceptability of personalised screening and primary prevention from a woman’s perspective. Our conceptual framework may offer guidance in this.
The studies on biomedical prevention, in particular those on chemoprevention, were mostly performed in the United States (US). Since the US does not have a population-based screening programme and the provision of prevention is more individualised, it is unclear whether these results are generalisable to a population-based screening setting. A review of studies with BRCA1 and BRCA2 mutation carriers has shown significant international variations in the rates of chemoprevention uptake, with higher rates in the US compared to, e.g., Europe [Citation79]. Future research is required to explore women’s perceptions of biomedical prevention in a breast cancer screening setting.
Conclusions
This review provides a conceptual framework which illustrates the factors that influence women’s acceptability of the adoption of personalised risk-based breast cancer screening and prevention. Women’s adoption appears to be based on a careful consideration of perceived susceptibility to breast cancer and a cost–benefit analysis of the potential health behaviours, taking into account perceived competence, autonomy, relatedness, and personal preference. To meet women’s needs in this decision-making process, significant changes in current practice are required. Developing evidence-based shared decision aids and family-oriented (genetic) counselling programmes will aid the transition from theory to practice.
Supplemental Material
Download Zip (100.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Pharoah PDP, Antoniou AC, Easton DF, et al. Polygenes, risk prediction, and targeted prevention of breast cancer. N Engl J Med. 2008;358:2796–2803.
- Vilaprinyo E, Forne C, Carles M, et al. Cost- effectiveness and harm-benefit analyses of risk-based screening strategies for breast cancer. Plos One. 2014;9:e86858.
- Rondy M, van Lier A, van de Kassteele J, et al. Determinants for HPV vaccine uptake in the Netherlands: a multilevel study. Vaccine. 2010;28(9):2070–2075.
- National Collaborating Centre for Cancer. Familial breast cancer: Classification and care of people at risk of familial breast cancer and management of breast cancer and related risks in people with a family history of breast cancer. Update of clinical guideline 14 and 41. Clinical guideline 164. 2013. London: NCCC. Available from: http://guidance.nice.org.uk/CG164
- Wilmot EG, Edwardson CL, Achana FA, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55:2895–2905.
- CASP. Critical appraisal skills programme (CASP): Making sense of evidence. 2011. Available from http://www.casp-uk.net/
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Borenstein M, Hedges LV, Higgins J, Rothstein HR. An introduction to meta-analysis. Hoboken, NJ: John Wiley & Sons, Ltd 2009.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
- Gorin SS, Wang C, Raich P, et al. Decision-making in cancer primary prevention and chemoprevention. Ann Behav Med. 2006;32(3):179–187.
- Henneman L, Timmermans DR, Bouwman CM, et al. ‘A low risk is still a risk’: exploring women’s attitudes towards genetic testing for breast cancer susceptibility in order to target disease prevention. Pub Health Genomics. 2011;14:238–247.
- Koitsalu M, Sprangers MA, Eklund M, et al. Public interest in and acceptability of the prospect of risk-stratified screening for breast and prostate cancer. Acta Oncol. 2016;55:45–51.
- Phelps C, Wood F, Bennett P, et al. Knowledge and expectations of women undergoing cancer genetic risk assessment: a qualitative analysis of free-text questionnaire comments. J Genet Couns. 2007;16:505–514.
- Meisel SF, Pashayan N, Rahman B, et al. Adjusting the frequency of mammography screening on the basis of genetic risk: attitudes among women in the UK. Breast. 2015;24:237–241.
- Wright CE, Harvie M, Howell A, et al. Beliefs about weight and breast cancer: an interview study with high risk women following a 12 month weight loss intervention. Hered Cancer Clin Pract. 2015;13:1–9.
- Lerman C, Rimer BK, Daly M, et al. Recruiting high risk women into a breast cancer health promotion trial. Cancer Epidemiol Biomarkers Prev. 1994;3:271–276.
- Heisey R, Pimlott N, Clemons M, et al. Women’s views on chemoprevention of breast cancer: qualitative study. Can Fam Physician. 2006;52:624–625.
- Bober SL, Hoke LA, Duda RB, et al. Decision-making about tamoxifen in women at high risk for breast cancer: clinical and psychological factors. J Clin Oncol. 2004;22:4951–4957.
- Port ER, Montgomery LL, Heerdt AS, et al. Patient reluctance toward tamoxifen use for breast cancer primary prevention. Ann Surg Oncol. 2001;8:580–585.
- Fasching PA, von Minckwitz G, Fischer T, et al. The impact of breast cancer awareness and socioeconomic status on willingness to receive breast cancer prevention drugs. Breast Cancer Res Treat. 2007;101:95–104.
- Altschuler A, Somkin CP. Women’s decision-making about whether or not to use breast cancer chemoprevention. Women Health. 2005;41:81–95.
- Dillard AJ, Ubel PA, Smith DM, et al. The distinct role of comparative risk perceptions in a breast cancer prevention program. Ann Behav Med. 2001;42:262–268.
- Meiser B, Butow P, Price M, et al. Attitudes to prophylactic surgery and chemoprevention in Australian women at increased risk for breast cancer. J Womens Health. 2003;12:769–778.
- Hallowell, N. ‘You don’t want to lose your ovaries because you think “I might become a man”’. Women’s perceptions of prophylactic surgery as a cancer risk management option. Psycho-oncol. 1998;7:263–275.
- Roetzheim RG, Lee JH, Fulp W, et al. Acceptance and adherence to chemoprevention among women at increased risk of breast cancer. Breast. 2015;24:51–56.
- Meiser B, Butow P, Friedlander M, et al. Intention to undergo prophylactic bilateral mastectomy in women at increased risk of developing hereditary breast cancer. J Clin Oncol. 2000;18:2250–2257.
- Salant T, Ganschow PS, Olopade OI, et al. ‘Why take it if you don’t have anything?’ Breast cancer risk perceptions and prevention choices at a public hospital. J Gen Intern Med. 2006;21:779–785.
- McKay A, Martin W, Latosinsky S. How should we inform women at higher risk of breast cancer about tamoxifen? An approach with a decision guide. Breast Cancer Res Treat. 2005;94:153–159.
- Karp J, Brown KL, Sullivan MD, et al. The prophylactic mastectomy dilemma: a support group for women at high genetic risk for breast cancer. J Genet Couns. 1999;8:163–173.
- Hatcher MB, Fallowfield LJ. A qualitative study looking at the psychosocial implications of bilateral prophylactic mastectomy. Breast. 2003;12:1–9.
- Donnelly LS, Evans DG, Wiseman J, et al. Uptake of tamoxifen in consecutive premenopausal women under surveillance in a high-risk breast cancer clinic. Br J Cancer. 2014;110:1681–1687.
- Holmberg C, Daly M, McCaskill‐Stevens W. Risk scores and decision-making: the anatomy of a decision to reduce breast cancer risk. J Nurs Healthc Chronic Illn. 2010;2(4):271–280.
- Fisher B, Dowding D, Pickett KE, et al. Health promotion at NHS breast cancer screening clinics in the UK. Health Promot Int. 2007;22:137–1145.
- Fang CY, Tseng M, Daly MB. Correlates of soy food consumption in women at increased risk for breast cancer. J Am Diet Assoc. 2005;105:1552–1558.
- Kaplan CP, Kim SE, Wong ST, et al. Willingness to use tamoxifen to prevent breast cancer among diverse women. Breast Cancer Res Treat. 2012;133:357–366.
- Julian‐Reynier CM, Bouchard LJ, Evans DG, et al. Women’s attitudes toward preventive strategies for hereditary breast or ovarian carcinoma differ from one country to another. Cancer 2001;92:959–968.
- Anderson, EE, Tejeda S, Childers K, et al. Breast cancer risk assessment among low-income women of color in primary care: a pilot study. J Oncol Pract. 2015;11:e460–e467.
- Razzaboni E, Toss A, Cortesi L, et al. Acceptability and adherence in a chemoprevention trial among women at increased risk for breast cancer attending the Modena Familial Breast and Ovarian Cancer Center (Italy). Breast J. 2013;19:10–21.
- Tjia J, Micco E, Armstrong K. Interest in breast cancer chemoprevention among older women. Breast Cancer Res Treat. 2008;108:435–453.
- Holmberg C, Waters EA, Whitehouse K, et al. My lived experiences are more important than your probabilities: the role of individualized risk estimates for decision-making about participation in the study of tamoxifen and raloxifene (STAR). Med Decis Making. 2015;35:1010–1022.
- Aktas B, Sorkin M, Pusztai L, et al. Uptake of exemestane chemoprevention in postmenopausal women at increased risk for breast cancer. Eur J Cancer Prev. 2016;25:3–8.
- Cyrus‐David MS, Strom SS. Chemoprevention of breast cancer with selective estrogen receptor modulators: views from broadly diverse focus groups of women with elevated risk for breast cancer. Psycho-oncology 2001;10(6):521–533.
- Daly M, Seay J, Balshem A, et al. Feasibility of a telephone survey to recruit health maintenance organization members into a tamoxifen chemoprevention trial. Cancer Epidemiol Biomarkers Prev. 1992;1:413–416.
- Fagerlin A, Zikmund-Fisher BJ, Smith DM, et al. Women’s decisions regarding tamoxifen for breast cancer prevention: responses to a tailored decision aid. Breast Cancer Res Treat. 2010;119:613–620.
- Melnikow J, Paterniti D, Azari R, et al. Preferences of women evaluating risks of tamoxifen(POWER): study of preferences for tamoxifen for breast cancer risk reduction. Cancer 2005;103:1996–2005.
- Yeomans-Kinney A, Vernon SW, Frankowski RF, et al. Factors related to enrollment in the breast cancer prevention trial at a comprehensive cancer center during the first year of recruitment. Cancer. 1995;76:46–56.
- Khazaee-Pool M, Montazeri A, Majlessi F, et al. Breast cancer preventive behaviors: exploring Iranian women’s experiences. BMC Womens Health. 2014;14:41–50.
- Dillard AJ, Scherer L, Ubel PA, et al. Breast cancer anxiety’s associations with responses to a chemoprevention decision aid. Soc Sci Med. 2013;77:13–19.
- Bastian LA, Lipkus IM, Kuchibhatla MN, et al. Women’s interest in chemoprevention for breast cancer. Arch Intern Med. 2001;161:1639–1644.
- Stefanek M, Enger C, Benkendorf J, et al. Bilateral prophylactic mastectomy decision-making: a vignette study. Prev Med. 1999;29:216–221.
- Lloyd SM, Watson M, Oaker G, et al. Understanding the experience of prophylactic bilateral mastectomy: a qualitative study of ten women. Psycho-oncol. 2000;9:473–485.
- Begum P, Richardson CE, Carmichael AR. Obesity in post-menopausal women with a family history of breast cancer: prevalence and risk awareness. Int Semin Surg Oncol. 2009;6:1–5.
- Rondanina G, Puntoni M, Severi G, et al. Psychological and clinical factors implicated in decision-making about a trial of low-dose tamoxifen in hormone replacement therapy users. J Clin Oncol. 2008;26:1537–1543.
- McLeish L, Reis MM, Stewart C, et al. Lifestyle changes in women at genetic risk of breast cancer: an observational study. Int J Behav Med. 2013;20:514–521.
- Spector D, Mishel M, Skinner CS, et al. Breast cancer risk perception and lifestyle behaviors among white and black women with a family history. Cancer Nurs. 2009;32:299–317.
- Grann VR, Patel P, Bharthuar A, et al. Breast cancer-related preferences among women with and without BRCA mutations. Breast Cancer Res Treat. 2010;119:177–184.
- Ozanne EM, Wittenberg E, Garber JE, et al. Breast cancer prevention: patient decision-making and risk communication in the high risk setting. Breast J. 2010;16:38–47.
- Unruh HK, Bowen DJ, Meischke H, et al. Women’s approaches to the use of new technology for cancer risk information. Womens Health. 2004;40:59–78.
- Stacey D, DeGrasse C, Johnston L. Addressing the support needs of women at high risk for breast cancer: evidence-based care by advanced practice nurses. Oncol Nurs Forum. 2002;29:77–84.
- Bernhardt BA, Geller G, Strauss M, et al. Toward a model informed consent process for BRCA1 testing: a qualitative assessment of women’s attitudes. J Genet Counsel. 1997;6:207–222.
- Papies EK. Health goal priming as a situated intervention tool: how to benefit from nonconscious motivational routes to health behaviour. Health Psychol Rev. 2016;10:408–424.
- Webb TL, Sheeran P. Does changing behavioral intentions engender behavior change? A meta-analysis of the experimental evidence. Psychol Bull. 2006;132:249–268.
- Evans DG, Brentnall AR, Harvie M, et al. Breast cancer risk in young women in the National Breast Screening Programme: implications for applying NICE guidelines for additional screening and chemoprevention. Cancer Prev Res. 2014;7(10):993–1001.
- Evans DGR, Donnelly LS, Harkness EF, et al. Breast cancer risk feedback to women in the UK NHS breast screening population. Br J Cancer. 2016;114(9):1045–1052.
- Hollands GJ, Marteau TM, Fletcher PC. Non-conscious processes in changing health-related behaviour: a conceptual analysis and framework. Health Psychol Rev. 2016;10(4):381–394.
- Harmsen MG, Hermens RP, Prins JB, et al. How medical choices influence quality of life of women carrying a BRCA mutation. Crit Rev Oncol Hematol. 2015;96(3):555–568.
- Howell A, Anderson AS, Clarke RB, et al. Risk determination and prevention of breast cancer. Breast Cancer Res. 2014;16(5):446.
- Harvie M, Cohen H, Mason C, et al. Adherence to a diet and exercise weight loss intervention amongst women at increased risk of breast cancer. Open Obes J. 2010;2:71–80.
- Kushi LH, Doyle C, McCullough M, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention. CA Cancer J Clinicians. 2012;62(1):30–67.
- van Asperen CJ, Tollenaar RA, Krol-Warmerdam EM, et al. Possible consequences of applying guidelines to healthy women with a family history of breast cancer. Eur J Hum Genet. 2003;11:633–667.
- Cuzick J, Sestak I, Bonanni B, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827–1834.
- Loehberg CR, Jud SM, Haeberle L, et al. Breast cancer risk assessment in a mammography screening program and participation in the IBIS-II chemoprevention trial. Breast Cancer Res Treat. 2010;121(1):101–110.
- Decensi A, Robertson C, Viale G, et al. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst. 2003;95(11):779–790.
- DeCensi A, Thorat MA, Bonanni B, et al. Barriers to preventive therapy for breast and other major cancers and strategies to improve uptake. Ecancermedicalscience 2015;9:595–607.
- Edwards AG, Naik G, Ahmed H, et al. Personalised risk communication for informed decision-making about taking screening tests. Cochrane Library. 2013.
- Trevena LJ, Zikmund-Fisher BJ, Edwards A, et al. Presenting quantitative information about decision outcomes: a risk communication primer for patient decision aid developers. BMC Med Inform Decis Mak. 2013;13:S7.
- Speice J, McDaniel SH, Rowley PT, et al. Family issues in a psychoeducation group for women with a BRCA mutation. Clin Genet. 2002;62(2):121–127.
- Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090–3095.
- Metcalfe KA, Birenbaum-Carmeli D, Lubinski J, et al. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. Int J Cancer. 2008;122(9):2017–2022.
- Miller WR, Rollnick S. Motivational interviewing: preparing people to change addictive behavior. New York: Guilford Press. 1991.
- Becker MH. The Health Belief Model and personal health behaviour. Health Education. 1974;2(3):324–508.
- Hagger MS, Wood C, Stiff C, et al. The strength model of self-regulation failure and health-related behaviour. Health Psychol Rev. 2009;3(2):208–238.
- Myers RE. Decision counseling in cancer prevention and control. Health Psychol. 2005;24:S71–S77.