Abstract
Background: Pattern of spread in patients with metastatic colorectal cancer (mCRC) is variable and may reflect different biology in subsets of patients. This is a retrospective study to explore the outcome of patients with mCRC based on their site of metastasis at diagnosis and to explore the association between tumor characteristics [KRAS/RAS, BRAF, mismatch repair (MMR) status, site of primary] and the site of metastasis.
Methods: Patients from two Australian databases were divided into six groups based on site of metastasis at time of diagnosis of metastatic disease; lung-only, liver-only, lymph node-only or any patients with brain, bone or peritoneal metastases. Primary endpoint was overall survival (OS) of each cohort compared with the rest of the population. A Mantel–Haenszel chi-squared test used to explore the association between site of metastasis and selected tumor characteristics.
Results: Five thousand nine hundred and sixty-seven patients were included. In a univariate analysis, median OS was significantly higher when metastases were limited to lung or liver and shorter for those with brain, bone or peritoneal metastases (p < .001) in both datasets. BRAF mutation was strongly associated with peritoneal metastases (relative risk = 1.8, p < .001) with lower incidence of lung (RR = 0.3, p = .004) and liver (RR = 0.7, p = .005) limited metastases. Lung-only metastases were more frequent with KRAS/RAS mutation (RR = 1.4, p = .007). Left colon tumors were associated with bone (RR = 1.6, p < .001) and lung-only metastases (RR = 2.3, p = .001) while peritoneal spread was less frequent compared with right colon tumors (RR = 0.6, p < .001). Rectal cancer was associated with brain, bone and lung metastases (RR = 1.7; p = .002, 1.7; p < .001, 2.0; p < .001). Liver-only metastases were less frequent in deficient MMR tumors (RR = 0.7, p = .01).
Conclusion: Survival duration with mCRC is related to the site of metastases with lung limited disease showing a more favorable survival outcome compared to other single metastatic site disease. The BRAF mutation and primary rectal cancer were associated with poor prognostic metastatic sites.
Introduction
Despite recent advances in early detection, systemic therapy, and localized treatment modalities, colorectal cancer remains one of the leading causes of cancer-related deaths and morbidity worldwide. In 2012, an estimated 694,000 deaths have occurred due to colorectal cancer worldwide [Citation1]. The prognosis of patients with metastatic colorectal without treatment is poor with an average life span of 5–6 months [Citation2].
Although the liver is the most common site for metastasis, it is recognized that different patients with colorectal cancer have distinctive patterns of spread. The number of metastases and tumor volume are strong predictor of prognosis [Citation3]. It remains inconclusive as to whether the anatomic site of the metastases has any prognostic implications [Citation3,Citation4]. Elias et al. [Citation3] reported no association between metastatic site and prognosis, whereas Carpizo et al. [Citation5] found significant association; however, both studies were limited by their small sample size. Survival of patients with lung-only colorectal cancer metastases appears to be at least comparable to survival with liver-only metastases, whereas the prognosis of patients with bone, peritoneal and cerebral metastases is much poorer [Citation6–8]. Two other studies looked at the survival pattern of patients with metastatic colorectal cancer, but one included many patients in the era before modern chemotherapy and other was a much smaller study [Citation9,Citation10]. It is plausible that different genotypes of metastatic colorectal cancer could present with different patterns of spread, malignant potential and prognosis. We conducted this study to explore the survival outcome of patients with metastatic colorectal cancer with different sites of metastasis at diagnosis.
In addition, it has been increasingly recognized that biological behavior and prognosis of colorectal cancer may vary according to the location of the primary, BRAF, KRAS and MMR (mismatch repair) status, though data are not consistent [Citation9,Citation11]. Some retrospective analyses suggest these somatic and germ line mutations may have an impact on the pattern of metastasis, but most of these are small, uncontrolled and single institutional studies with varying associations [Citation12,Citation13]. The second part of this study was to investigate any association between molecular markers, site of primary and site of metastasis.
Methods
This is a retrospective study of two different data sets extracted from two large Australian databases. The South Australian (SA) Cancer Registry [Citation14] is a state-wide population based registry which captures all incident cases, while ‘Treatment of Recurrent and Advanced Colorectal Cancer’ (TRACC) registry [Citation15] is a federated national database with data collected from participating cancer centers in the Australian Capital Territory, Victoria and Tasmania. The latter only includes patients referred to these participating cancer centers for treatment. Both data registries store data in a de-identified form and have existing ethics approvals from relevant human research ethics committees.
The primary objective was to characterize the natural history of patients with metastatic colorectal cancer based on individual sites of metastasis. Secondary objective was to explore any association between molecular markers, site of primary with site of metastasis. Molecular markers included were MMR, BRAF and KRAS/RAS mutational status.
Patient with proven metastatic colorectal cancer who were registered on either database from January 2005 to December 2015 were included. Survival was calculated from date of diagnosis of metastatic cancer to the date of death. Patients alive at the date of last contact were censored in the analysis. Survival cutoff was June 2016. Cases were divided into six cohorts depending on the site of metastasis at the diagnosis of metastatic cancer; three of which included only patients with single sites of metastatic disease (liver-only, lung-only, lymph node-only metastases). The remaining three cohorts included any patients with brain metastases, peritoneal metastases or bone metastases. Overall survival of each cohort was compared with over-all survival for the rest of the population (ROP). ROP in each cohort was defined as all patients who did not have metastasis in the corresponding organ and/or those with >1 site of metastases. For example, in the lung-only cohort, ROP included patients who did not have lung metastasis and those with >1 site of metastasis.
The overall survival data for each cohort was compared with that of the overall population using Kaplan–Meier analysis and the log rank test. Due to potential differences in the patient populations and methods of data collection, we analyzed the survival data from the two databases separately and compared them. The SA cohort with larger numbers was the primary dataset and TRACC dataset was used to validate the results from the SA cohort. ‘Program R’, version 3.4.3 and SAS Enterprise Guide Version 6.1 were used for the statistical analyses. A two-sided p value of <.05 was considered statistically significant.
The site of the colorectal primary and results of routine testing for the molecular markers (including KRAS/RAS, BRAF and MMR) were collected. The association of these factors with the site of metastases was assessed with Mantel–Haenszel chi-squared test on the pooled data from both data sets.
Results
We identified a total of 5967 patients with metastatic colorectal cancer from both databases (SA = 4182; 58% male, TRACC = 1785; 57% male). Median age was 70 and 68 years in SA and TRACC data sets, respectively. The majority of patients in the TRACC database had good performance status of ECOG 0–1 while these data were not available in SA database. Sixty-one percent of patients in the SA cohort and 78% of patients in the TRACC cohort had received at least one line of systemic treatment for metastatic disease (). The rate of metastasectomy was 20% in SA and 30% in TRACC dataset. After a median follow-up of 12 and 34 months there were 3172 and 1002 deaths in SA and TRACC cohort, respectively.
Table 1. Chemotherapy data.
Survival analysis
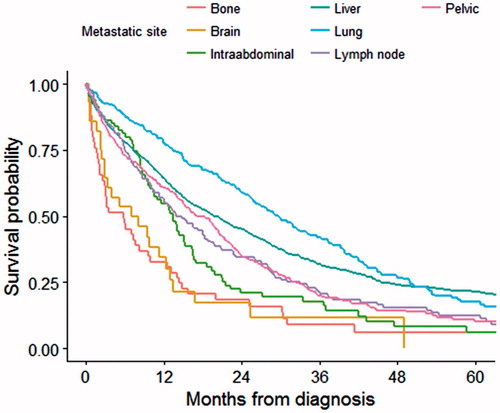
The median survivals of the overall population were 14.9 and 25.1 months for the SA and TRACC datasets, respectively. In the small proportion of patients where lungs were the only site of metastasis (8.7%), the median overall survival was significantly longer at 29.5 months in the SA [hazard ratio (HR) = 0.61, 95% CI 0.53–0.69, p < .0001] dataset and 39 months in the TRACC dataset (HR = 0.41, 95% CI 0.32–0.52, p < .0001), compared to the rest of the population. Overall 36% of patients had metastasis confined to the liver; their median overall survival was significantly higher, 19.6 and 30 months in the SA (HR = 0.67, 95% CI 0.62–0.72, p = .0001) and TRACC (HR = 0.57, 95% CI 0.50–0.66, p < .0001) datasets respectively compared to rest of the population. The median overall survival of patients with any brain metastasis, any bone metastasis and any peritoneal metastasis in the SA dataset were 5.8, 6.7 and 12.4 months, respectively, and 6, 12 and 17 months in the TRACC dataset. These survival figures were statistically lower than for the rest of the population in the SA and TRACC dataset (p < .0001 in each cohorts). Survival of patients with lymph node-only metastasis was significantly higher in TRACC dataset compared with rest of the population; however there was no difference in SA dataset ( and ). A multivariate analysis was performed in SA dataset and the survival association remained significant in all the cohorts except for liver and peritoneal cohorts (). When patients with single-organ metastatic disease were compared with each other, the differences in overall survival was statistically significant (p < .0001) ().
Table 2. Overall survival: SA cohort.
Table 3. Overall survival: TRACC cohort.
Molecular markers and site of metastasis
BRAF, KRAS/RAS and MMR (MSI) data were available for 20, 37 and 21% of the overall population (Supplementary Tables S1 and S2). Presence of BRAF mutation was associated with low incidence of liver or lung-limited disease with a relative risk (RR) of 0.7 (p = .005) and 0.3 (p = .004), respectively. BRAF-mutated colorectal cancer showed higher incidence of peritoneal metastasis (RR = 1.8, p < .001) and lower incidence of liver (RR = 0.7, p = .005) and lung (RR = 0.3, p = .004) limited disease. Although incidence of cerebral metastasis was 1.4 times higher in BRAF mutant patients than BRAF wild-type patients, this was not statistically significant (p = .8) ().
Table 4. Regression model estimates for predictors (adjusted for age >65 years, sex, site of primary, metastasectomy) in South Australian dataset.
Table 5. Asociation between KRAS, BRAF mutation, dMMR, primary site and site of metastasis.
Lung-only metastases were more likely in patients with KRAS/RAS mutant metastatic colorectal cancer (RR = 1.4, p = .007), while the incidence of lymph node-only metastasis was significantly lower (RR = 0.34) in those with KRAS/RAS mutation. No association was seen with other sites of metastasis and KRAS status. Liver-only metastases were significantly less frequent in deficient MMR (dMMR) tumors (RR = 0.7, p = .01) though MMR status was not associated with other site of metastases ().
Site of primary and site of metastasis
Left-sided colon tumors were associated with significantly higher rates of lung-only metastases (RR = 2.3, p = .001) or any bone metastases (RR = 1.6, p < .001) while peritoneal spread was less frequent compared with right colon tumors (RR = 0.6, p < .001). Rectal cancer was more frequently associated with lung-only (RR = 2.0, p < .001), brain (RR = 1.7, p = .002), and bone (RR = 1.7, p < .001) metastases compared with rest of the left colon primaries ().
Discussion
Clinicopathological stage of the cancer at diagnosis is the single most important determinant of prognosis, but there are other factors which influence the outcome of patients. Molecular markers like BRAF [Citation16] and KRAS mutation [Citation17], MMR status [Citation18], staining for CDX2 [Citation19], 18q deletion [Citation20], and other features like pre-operative carcinoembryonic antigen (CEA) [Citation21] and, tumor volume [Citation3] have been implicated in the prognosis of colorectal cancer and sometimes in prediction of response to therapy. Immune profiling and immunoscore may become a prognostic as well as a predictive tool, especially for immunotherapy; however, it is not widely utilized currently in clinical practice until further validation [Citation22].
Survival outcome depending on site of metastasis
There was strong association found between survival outcome and site of metastasis. The association remained significant in the SA dataset when adjusted in a multivariate model for age, sex, and site of primary, except for liver and peritoneal cohort, potentially due to the differences in the rate of metastasectomy. Consistent with the literature [Citation6,Citation7,Citation23,Citation24], the survival of brain, peritoneal and bone metastases were poor. The effect on critical functions from brain metastases and debilitating local complications from peritoneal metastases could explain these results, but it is not understood why bone metastases carry such poor prognosis. Furthermore, comparison of survival outcome of single-site metastatic disease revealed significant difference with the lung-only cohort showing the best survival, while the brain and bone cohort had the worst outcome. This pattern was very similar in both datasets. Hence, the question arises as to why only subsets of patients with metastatic colorectal cancer have metastases in these sites and have differential biological behavior? Is it purely a mechanical lodgment of cancer cells as proposed by Ewing [Citation25] or could it be explained by the inherent variations in cancer biology? In the current study, strong associations were seen in between site of metastasis and some of the molecular markers, which is discussed below. This is hypothesis generating and future studies are needed to confirm these associations.
Although median survival was numerically higher in the TRACC dataset compared to that of SA, the pattern of survival according to metastatic sites was very similar, despite potential differences in the methodology of data-collection in each dataset ( and ). As noted earlier, the SA Registry is a population-based registry which captures all the cases of colorectal cancer diagnosed in the state, where as TRACC is a tertiary hospital-based registry established in late 2009. The SA registry captures all incident cases regardless of comorbidity, performance status and referral patterns. This registry includes cases that have not been referred to Medical Oncology or Surgical Departments of major tertiary referral centers. This database represents a thorough all-inclusive case-based experience of a given population. Hospital-based registries generally have more direct access to clinical data and tend to be more comprehensive with less delay in data collection than population-based registries [Citation26]. Because they are institution-based registries involving only participating centers, they do not enter all index cases. On the other hand, ascertainment of death is likely to be more complete in the population-based registries due to accessibility to other information sources. While these factors might have contributed to the numerical differences in the survivals observed in each population, the consistency of the survival patterns adds strength to the study results. This is further emphasized by the varied survival pattern seen in the literature; for example, median survival of those with liver only metastasis was 9 months in study by Riihimaki et al. [Citation9]. Padman et al. [Citation27] reported a 15 months median survival for patients with liver only metastasis who only had chemotherapy.
Metastatic pattern
BRAF mutation
In this study, BRAF mutation correlated positively with incidence of peritoneal metastasis and negatively with liver-only and lung-only metastasis. Poor survival outcome of BRAF mutation could be related to its propensity to metastasize frequently to poor prognostic sites like peritoneum and less frequently to liver or lung as seen in this study. However, incidence of peritoneal metastasis in BRAF mutant cancers shows wide variability, ranging from 10 to 45% [Citation12,Citation28]. Although there was no significant association seen between BRAF mutation, bony metastases and brain metastases, the numbers were too small to see any meaningful association.
Site of primary
Left-sided primaries seem to have better survival, response to treatment and differ in their molecular profile compared to right-sided primaries [Citation29,Citation30]. In our study, the incidence of lung-only metastases and bony metastases were significantly higher in left-sided primary cancers, though almost half those patients (45%) were of rectal origin (). The high incidence of brain, bone and lung-only metastases in rectal primaries is not fully explicable. However, in addition to the widely known ‘seed and soil’ theory [Citation31], this pattern could also be explained by the anatomical extra peritoneal location of rectum [Citation32].
KRAS mutation
The prognostic significance of the KRAS mutation is controversial and not well-established [Citation33,Citation34]. In our study, the incidence of lung-only metastases was higher with KRAS mutation in whom the response to EGFR-directed therapy is poor. Other retrospective studies have found similar associations between KRAS mutation and pulmonary metastasis [Citation35,Citation36]. Kemeny et al. [Citation37] reports higher recurrence rate after hepatic metastasectomy in patients with KRAS mutation compared to KRAS WT.
MMR status
The significance of dMMR phenotype in Stage IV disease is still uncertain [Citation28,Citation38]. In this study, dMMR tumors were inversely associated with liver-only metastases (), a finding that has been reported in some studies [Citation39–41]. Catalano et al. [Citation42] found non-mucinous tumors to be associated with more liver metastasis compared to mucinous counterpart and most dMMR tumors are phenotypically mucinous. Emerging data suggest cancer subtypes may possess different metastatic potential, tumor–stroma interaction, adhesive molecules and individualized chemokine secretion [Citation43,Citation44]. Further research is needed to fully characterize the dMMR phenotype.
Limitations and strengths
We acknowledge there were limitations to our study. Firstly, being a retrospective study there is likelihood of selection bias, information bias, and differences in method of data collection and follow-up, especially considering the fact that one registry was population based and other being hospital based (established in 2009). As noted earlier there could exist other unadjusted variables like tumor burden, and local ablative therapies which may have affected the survival outcome. However, analysis of survival separately in each dataset might have eliminated some of these potential biases. Secondly limitation was, low number of available molecular data. While missing data in the databases could be a factor for this, MMR, KRAS and BRAF status were also not universally available as testing for these mutations was not routine 10 years ago. The methods of testing have varied geographically and over time; for example, KRAS exon 2 mutation test was introduced in Australia from September 2010 via a pharmaceutical company access program, availability of which was variable geographically until 2011 when it was funded nationwide. KRAS exon 3, 4 and NRAS testing became available only in 2013 again via an access program and access was limited until 2014, when it was funded by the government. Another limitation is, it is possible that a single patient may have had more than one molecular markers in our dataset, which could interact with each other and may have affected the outcome.
The strength of our study was the size of the population. Analyzing two datasets separately and comparing them adds weight to the information derived, especially similarities in the pattern of survival outcome.
In conclusion, our study observed a more favorable survival outcome in patient with metastatic colorectal cancer with metastases which were limited to liver or lungs and adverse outcome with brain, bone or peritoneal involvement. There were also significant variations in the survival among single-organ metastatic sites of disease. Presence of molecular markers like BRAF, KRAS mutation, MSI status and knowledge of site of primary cancer may give important clues regarding likely sites of early recurrence. This knowledge may help the physician in choosing appropriate targeted investigations and hence early diagnosis of recurrence. Our findings add to the knowledge of metastatic sites and differential survival pattern, impact of molecular markers and site of primary cancer. Further research into tumor microenvironment and characterization of molecular markers may explain the associations found in our study and might improve the concept of personalized medicine.
Supplemental Material
Download MS Word (21.5 KB)Acknowledgments
Roche Products Pty Limited has provided financial assistance for the development, installation and maintenance of the BioGrid-TRACC registry. The South Australian State Government has provided support for the maintenance of the SA Metastatic Colorectal Cancer Registry. All authors listed have made substantial, direct and intellectual contribution in the design, acquisition of data, conduct, writing and editing of this article.
Disclosure statement
P.G. has received honoraria from SIRTEX, Amgen, Bayer, Roche, Servier and Merck. D.Y. has received honoraria from Roche, Amgen, BMS and Merck. Others have declared no conflicts of interest.
References
- International Agency for Research on Cancer (IARC) [Internet]. GLOBOCAN 2012: colorectal cancer estimated incidence, mortality and prevalence worldwide in 2012. France: IARC; 2012. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer=colorectal
- Scheithauer W, Rosen H, Kornek GV, et al. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ. 1993;306:752–755.
- Elias D, Liberale G, Vernerey D, et al. Hepatic and extrahepatic colorectal metastases: when resectable, their localization does not matter, but their total number has a prognostic effect. Ann Surg Oncol. 2005;12:900–909.
- Pulitano C, Bodingbauer M, Aldrighetti L, et al. Liver resection for colorectal metastases in presence of extrahepatic disease: results from an international multi-institutional analysis. Ann Surg Oncol. 2011;18:1380–1388.
- Carpizo DR, Are C, Jarnagin W, et al. Liver resection for metastatic colorectal cancer in patients with concurrent extrahepatic disease: results in 127 patients treated at a single center. Ann Surg Oncol. 2009;16:2138–2146.
- Nozue M, Oshiro Y, Kurata M, et al. Treatment and prognosis in colorectal cancer patients with bone metastasis. Oncol Rep. 2002;9:109–112.
- Koppe MJ, Boerman OC, Oyen WJ, et al. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243:212–222.
- Damiens K, Ayoub JP, Lemieux B, et al. Clinical features and course of brain metastases in colorectal cancer: an experience from a single institution. Curr Oncol. 2012;19:254–258.
- Riihimaki M, Hemminki A, Sundquist J, et al. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6:29765.
- Khattak MA, Martin HL, Beeke C, et al. Survival differences in patients with metastatic colorectal cancer and with single site metastatic disease at initial presentation: results from South Australian clinical registry for advanced colorectal cancer. Clin Colorectal Cancer. 2012;11:247–254.
- Augestad KM, Bakaki PM, Rose J, et al. Metastatic spread pattern after curative colorectal cancer surgery. A retrospective, longitudinal analysis. Cancer Epidemiol. 2015;39:734–744.
- Cho M, Akiba C, Lau C, et al. Impact of RAS and BRAF mutations on carcinoembryonic antigen production and pattern of colorectal metastases. World J Gastrointest Oncol. 2016;8:128–135.
- Kim MJ, Lee HS, Kim JH, et al. Different metastatic pattern according to the KRAS mutational status and site-specific discordance of KRAS status in patients with colorectal cancer. BMC Cancer. 2012;12:347.
- Neo EL, Beeke C, Price T, et al. South Australian clinical registry for metastatic colorectal cancer. ANZ J Surg. 2011;81:352–357.
- Field K, Wong HL, Shapiro J, et al. Developing a national database for metastatic colorectal cancer management: perspectives and challenges. Intern Med J. 2013;43:1224–1231.
- Tie J, Gibbs P, Lipton L, et al. Optimizing targeted therapeutic development: analysis of a colorectal cancer patient population with the BRAF(V600E) mutation. Int J Cancer. 2011;128:2075–2084.
- Yoon HH, Tougeron D, Shi Q, et al. KRAS codon 12 and 13 mutations in relation to disease-free survival in BRAF-wild-type stage III colon cancers from an adjuvant chemotherapy trial (N0147 alliance). Clin Cancer Res. 2014;20:3033–3043.
- Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7:153–162.
- Dalerba P, Sahoo D, Paik S, et al. CDX2 as a prognostic biomarker in stage II and stage III colon cancer. N Engl J Med. 2016;374:211–222.
- Carethers JM, Hawn MT, Greenson JK, et al. Prognostic significance of allelic lost at chromosome 18q21 for stage II colorectal cancer. Gastroenterology. 1998;114:1188–1195.
- Park IJ, Choi GS, Lim KH, et al. Serum carcinoembryonic antigen monitoring after curative resection for colorectal cancer: clinical significance of the preoperative level. Ann Surg Oncol. 2009;16:3087–3093.
- Galon J, Costes A, Sanchez CF, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964.
- Vatandoust S, Price TJ, Karapetis CS. Colorectal cancer: metastases to a single organ. World J Gastroenterol. 2015;21:11767.
- Kruser TJ, Chao ST, Elson P, et al. Multidisciplinary management of colorectal brain metastases: a retrospective study. Cancer. 2008;113:158–165.
- James E. Neoplastic diseases: a treatise on tumors. 3rd ed. Philadelphia: W.B. Saunders; 1928.
- Silva IS, editor. The role of cancer registries. Cancer epidemiology: principles and methods. Lyon: International Agency for Research on Cancer; 1999.
- Padman S, Padbury R, Beeke C, et al. Liver only metastatic disease in patients with metastatic colorectal cancer: impact of surgery and chemotherapy. Acta Oncol. 2013;52:1699–1706.
- Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–4632.
- Petrelli F, Tomasello G, Borgonovo K, et al. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol. 2016 [Oct 27]. DOI:10.1001/jamaoncol.2016.4227
- Venook AP, Niedzwiecki D, Federico I, et al. Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol. 2016;34:3504.
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101.
- Mitry E, Guiu B, Cosconea S, et al. Epidemiology, management and prognosis of colorectal cancer with lung metastases: a 30-year population-based study. Gut. 2010;59:1383–1388.
- Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–474.
- Yaeger R, Cowell E, Chou JF, et al. RAS mutations affect pattern of metastatic spread and increase propensity for brain metastasis in colorectal cancer. Cancer 2015;121:1195–1203.
- Tie J, Lipton L, Desai J, et al. KRAS mutation is associated with lung metastasis in patients with curatively resected colorectal cancer. Clin Cancer Res. 2011;17:1122–1130.
- Ghidini M, Personeni N, Bozzarelli S, et al. KRAS mutation in lung metastases from colorectal cancer: prognostic implications. Cancer Med. 2016;5:256–264.
- Kemeny NE, Chou JF, Capanu M, et al. KRAS mutation influences recurrence patterns in patients undergoing hepatic resection of colorectal metastases. Cancer. 2014;120:3965–3971.
- Goldstein J, Tran B, Ensor J, et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H). Ann Oncol. 2014;25:1032–1038.
- Fujiyoshi K, Yamamoto G, Takenoya T, et al. Metastatic pattern of stage IV colorectal cancer with high-frequency microsatellite instability as a prognostic factor. Anticancer Res. 2017;37:239–247.
- Alvarado-Bachmann R, Smith A, Gundara JS, et al. The incidence of mismatch repair gene defects in colorectal liver metastases. Mol Med Rep. 2014;10:1003–1006.
- Haddad R, Ogilvie RT, Croitoru M, et al. Microsatellite instability as a prognostic factor in resected colorectal cancer liver metastases. Ann Surg Oncol. 2004;11:977–982.
- Catalano V, Loupakis F, Graziano F, et al. Mucinous histology predicts for poor response rate and overall survival of patients with colorectal cancer and treated with first-line oxaliplatin- and/or irinotecan-based chemotherapy. Br J Cancer. 2009;100:881–887.
- Itatani Y, Kawada K, Inamoto S, et al. The role of chemokines in promoting colorectal cancer invasion/metastasis. Int J Mol Sci. 2016;17:643.
- Schluter K, Gassmann P, Enns A, et al. Organ-specific metastatic tumor cell adhesion and extravasation of colon carcinoma cells with different metastatic potential. Am J Pathol. 2006;169:1064–1073.