Abstract
Purpose: To report acute and late genitourinary (GU) and gastrointestinal (GI) toxicities associated with post-prostatectomy proton therapy (PT).
Methods: The first 100 consecutive patients from 2010 to 2016 were retrospectively assessed. Baseline characteristics, prospectively graded CTCAE v4.0 toxicities, and patient-reported outcomes were reported. Late outcomes were reported for 79 patients with 3 months minimum follow up. Toxicity-free survival Kaplan-Meier curves were estimated. Logistic regression assessed associations between toxicities and clinical and treatment characteristics (p < .05 significance).
Results: Median age, months after surgery, and months of follow-up were respectively 64 years (range 42–77), 25 (5–216), and 25 (0–47). PT received was 70.2 Gy (RBE) (89%), salvage (93%), prostate bed only (80%), pencil beam scanning (86%), with IMRT (31%), and with androgen deprivation (34%). Acute and late maximum toxicities, respectively were: GU grade 0 (14%; 18%), 1 (71%; 62%), 2 (15%; 20%), ≥3 (0), and GI: grade 0 (66%; 73%), 1 (34%; 27%), ≥2 (0). Toxicity-free survival at 24 months was GU grade 2 (83%) and GI grade 1 (74%). Mean (±std dev) baseline International Prostate Symptom Score (IPSS), International Index of Erectile Function, and Expanded Prostate Cancer Index Composite bowel function and bother were 6.6 ± 6.1, 10.5 ± 7.3, 90.9 ± 10.8, 93.3 ± 11.2, respectively, and largely unchanged at 2 years: 6.3 ± 3.6, 11.1 ± 6.3, 92.8 ± 5.8, and 90.9 ± 10.3. On multivariate analysis, baseline IPSS (p = .009) associated with GU grade 2 acute toxicity. Bladderless-CTV median dose, V30, and V40 associated with GU grade 2 acute toxicity and maximum dose with late (Ps <0.05). For GI, on multivariate analysis, baseline bowel function (p = .033) associated with acute grade 1 toxicity. Rectal minimum and median dose, V10, and V20, and anterior rectal wall median dose and V10 through V65 associated with acute grade 1 GI toxicity (Ps < .05).
Conclusions: Post-prostatectomy PT for prostate cancer is feasible with a favorable GU and GI toxicity profile acutely and through early follow up.
Introduction
The role of radiotherapy (RT) after prostatectomy for prostate cancer is increasingly defined [Citation1]. Adjuvant randomized trials have demonstrated improvements in biochemical relapse-free survival and overall survival for certain patients, generally at the expense of mild to moderate increased toxicity [Citation2–4]. In the salvage setting, numerous retrospective series have demonstrated biochemical and overall survival benefits to salvage RT in specific subsets of patients [Citation5, Citation6], and prospective, randomized investigation by cooperative groups have recently demonstrated improved survival endpoints with the addition of concurrent androgen deprivation therapy (ADT) [Citation7, Citation8]. The additive acute and late treatment morbidities of RT in these settings are generally of concern in this patient population with increased underlying urinary dysfunction post-prostatectomy [Citation9], which may impede referral for consideration of therapy. As national trends move toward increasing use of surgery in high-risk disease [Citation10], the role of post-prostatectomy RT is expected to increase, which merits the investigation of methods to improve the therapeutic ratio and reduce integral dose exposure for these relatively younger patients that may carry significant lifetime risks from radiation [Citation11].
Proton therapy (PT) is an established treatment modality for intact prostate cancer [Citation12, Citation13] given its favorable physical properties of finite dose range and virtual lack of exit dose. These dosimetric advantages translate to reductions in integral, low, and intermediate dose regions compared to intensity modulated photon RT (IMRT) [Citation14, Citation15] but have not yet been prospectively demonstrated to translate into relative reductions in toxicity. While both a randomized and pragmatic trial are underway to examine this question, clinical outcomes with post-prostatectomy PT have not yet been reported to our knowledge. A prospective study of 10 patients undergoing post-prostatectomy IMRT used serial magnetic resonance imaging (MRI) to assess interfraction motion and target volume variability throughout the treatment course, suggesting feasibility of PT with appropriate motion management and patient alignment methods [Citation16]. The purpose of our study was to report the acute and late genitourinary (GU) and gastrointestinal (GI) toxicities for the first 100 patients undergoing post-prostatectomy PT at our institution and to assess toxicity associations with clinical and treatment parameters.
Methods and materials
We conducted an institutional review board–approved retrospective analysis of the first 100 patients undergoing post-prostatectomy PT at a single institution from 2010 to 2016.
Clinical evaluation
Patients had histologically confirmed, non-metastatic prostate adenocarcinoma status post radical prostatectomy and were generally evaluated by complete history, physical examination, bone scintigraphy, and computed tomography (CT) of the abdomen and pelvis and/or pelvic MRI ± endorectal coil. Concurrent elective pelvic nodal irradiation, classically termed whole pelvis (WP), and/or ADT, consisting of luteinizing hormone-releasing hormone analog administration initiated prior to PT, were at the physician’s discretion and offered primarily to patients with adverse risk features (PSA >10 ng/mL, T3a/T3b, and/or Gleason ≥7). Patients were monitored weekly during treatment and generally seen 3 months after RT completion and every 6 months thereafter. Maximum GU and GI graded toxicities were prospectively scored using Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [Citation17] during treatment and follow-up; erectile dysfunction and constipation were excluded and recorded separately. Patient-reported outcomes including the International Prostate Symptom Score (IPSS) (range of score 0–35; low score best) and quality of life bother score (range of score 0–6; low score best) [Citation18], International Index of Erectile Function (IIEF-5) (range of score 5–25; high score best) [Citation19], and Expanded Prostate Cancer Index Composite (EPIC) bowel function and bother scores (range of scores 0–100; high scores best) [Citation20] were recorded at initial consultation and each follow up visit. Late outcomes were reported for the 79 patients with minimum 3 months follow up.
RT planning and delivery
Planning CT was performed on a CT simulator (Siemens, NY, USA). Pre-CT preparation were advised to achieve a full bladder and empty rectum as previously described [Citation21]. Patients were immobilized supine with Knee-Lok and Foot-Lok cushions (CIVCO, Orange City, IA, USA), and an indexed lumen endorectal balloon (ERB) (ImmobiLoc, RadiaDyne, LLC, Houston, TX, USA) was inserted into the rectum and filled with 100 mL of water. A 1.5-mm slice scan was acquired at pelvic level and isocenter placed in the geometric center of the prostate bed (PB). The PB ± WP were delineated as the clinical target volume(s) (CTV) according to consensus guidelines [Citation22, Citation23]. PT and IMRT treatment plans were generated using Eclipse Treatment Planning (Software version 10; Varian, Palo Alto, CA, USA). To account for proton beam range uncertainty, a margin in the beam direction of 3.5% of the beam range was applied to correct for uncertainty in conversion from Hounsfield unit to proton stopping power with an additional 1 mm margin to correct for beam calibration uncertainty. For treatment plan optimization purposes for pencil beam scanning, a pencil beam scanning target volume was created to correct upfront for these uncertainties [Citation24]. The PB planning target volume (PTV) was created as a 10-mm uniform expansion, except 6-mm posteriorly, from the CTV, with the expansion based on lateral uncertainty alone as recommended by ICRU 78 [Citation25], and used for recording and reporting purposes. For the WP PTV, a 5–8 mm uniform expansion was similarly utilized. Minimum CTV and PTV coverage was D98 > 98% and D95 > 95%, respectively. All nominal plans were normalized to the mean dose of the CTV for comparison purposes. Prescription doses to the WP and PB CTVs were 50.4 and 66.6–70.2 Gy (RBE) in 1.8 Gy (RBE) fractions; one patient received an additional PB boost for gross recurrence to 75.6 Gy (RBE). Organs at risk (OAR) evaluated were contoured as previously described [Citation17]: bladder, bladderless-CTV (bladder minus CTV), rectum, infield rectum (rectum 1 cm above and below CTV), anterior rectal wall (ARW), and bowel cavity (small and large bowel) for cases with bowel proximal to the treatment field. Predefined institutional volumetric target dose constraints included bladderless-CTV volume receiving 65 Gy (V65) < 20%, V50 < 45%, and V45 < 50%, infield rectum V60 < 20%, V45 < 40%, and V40 < 50%, and bowel V45 < 175 cc. When target coverage and dose constraints were unachievable with PT alone, combined IMRT plans were used to achieve dose constraints. OAR DVHs were reviewed. VMAT plans were composed of two 6 MV coplanar arcs and treated on Varian linear accelerators (Varian 2300IX; Varian Medical Systems, Palo Alto, CA, USA). During PB PT, setup accuracy was verified for patients with daily kV-kV imaging with matching to the ERB filled with 10 cc diluted contrast to align the PB CTV and ARW (); daily cone beam CT was used during PB IMRT with similar alignment instruction (without the diluted contrast). During the WP course, daily kV-kV imaging and/or CBCT with bony pelvis alignment were utilized.
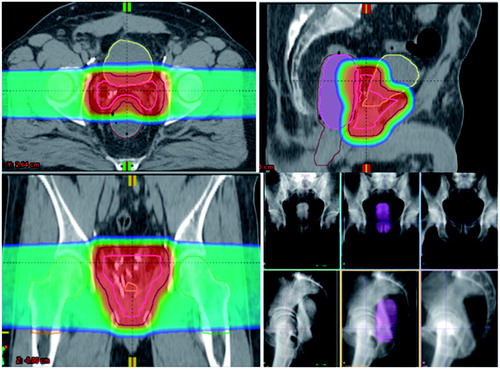
Figure 1. Representative dose color-wash distribution for post-prostatectomy PT using opposed lateral beams—contours: CTV (pink), PTV (navy), bladder (yellow), rectum (brown), right femoral head (orange), left femoral head (green), and ERB (purple). Daily imaging and alignment technique using the ERB with diluted contrast for visualization and alignment of the ARW with the PB CTV on daily kV-kV imaging.
Statistical analysis
Baseline patient characteristics and the frequency and type of maximum crude acute and late GU and GI graded toxicities were descriptively reported. Toxicity-free survival curves were estimated by the Kaplan-Meier product–limit method with the event of interest as the occurrence of grade 2 late toxicity for GU, and grade 1 late toxicity for GI; patients without grade 2 for GU and grade 1 for GI were censored. Logistic regression analysis was used to investigate separately the association between grade 2 GU acute and late toxicity and (1) clinical characteristics shown in , including age, prostate specific antigen (PSA), pathologic tumor stage, Gleason score, hypertension (HTN), hyperlipidemia, diabetes mellitus (DM), pre-RT GU PMH (GU past medical history defined as an antecedent history of significant urinary sign or symptoms, including benign prostatic hyperplasia, prior transurethral resection of the prostate, bladder outlet obstruction, and/or lower urinary tract symptoms), IPSS, IPSS bother score, modality type, dose, field size, and ADT and (2) bladder and bladderless-CTV dosimetric parameters. Similarly, associations were investigated separately between grade 1 GI acute and late toxicity and (1) the aforementioned clinical characteristics, adding EPIC bowel scores and excluding IPSS, IPSS bother score, and pre-RT GU PMH and (2) rectal (rectum, infield rectum, and ARW) dosimetric parameters. Markov Chain Monte Carlo multiple imputation procedure was used for missing data assuming missing at random and joint multivariate normal distribution. The parameters with >10% missing data (range 17–25%) were pre-operative PSA and the following patient reported outcomes (IPSS bother, IIEF, and EPIC bowel function and bother score). Patients with and without missing data did not differ in observed variables. Patient reported outcomes were assessed as continuous variables. Clinical characteristics with univariate toxicity associations (p < .05) were considered in a multivariate logistic regression model. A p value of <.05 was considered statistically significant. Statistical analyses were conducted using Stata version 14 software (StataCorp, College Station, TX, USA).
Table 1. Post-prostatectomy PT cohort baseline demographic, clinical, and treatment characteristics.
Results
summarizes the cohort characteristics. Seven patients were treated in the adjuvant setting with an undetectable ultrasensitive PSA (<0.01, <0.05, and <0.1); the remaining 93 patients had a detectable PSA and thus treated with salvage radiation: 27 had a PSA between 0.03 and 0.19, and the remaining 66 patients had a PSA >0.2. Fourteen of the 80 (17.5%) cases treated to the PB only and 17 of the 20 (85%) WP cases utilized combined PT and IMRT; the remaining received PT for the entire course. shows the mean dose volume histogram curves for target volumes and OAR. Median follow up was 24.5 months (range 0–47). shows the acute and late GU and GI toxicities.
Table 2. Acute and late maximum GU and GI CTCAE v 4.0 toxicities for post-prostatectomy PT.
GU toxicity
Acute maximum GU toxicity was grade 0 (14%), 1 (71%), and 2 (15%). There were no grade ≥3 toxicities. Acute grade 2 GU toxicities () consisted of worsened urinary: incontinence (11%), frequency (3%), retention (3%), urgency (2%), and tract pain (1%), and were generally managed with pharmacologic therapy. On univariate analysis, baseline IPSS (OR 1.089, 95% CI: 1.006, 1.179, p = .034) and IPSS bother score (OR 1.654, 95% CI: 1.166, 2.348, p = .005) were associated with acute grade 2 GU toxicity, and on multivariate analysis, only baseline IPSS bother score (OR 1.586, 95% CI: 1.076, 2.338, p = .020) remained significantly associated while baseline IPSS did not (OR 1.029, 95% CI: 0.937, 1.130, p = .550).
Table 3. Frequency of specific maximum GU grade 2 toxicities with post-prostatectomy PT.
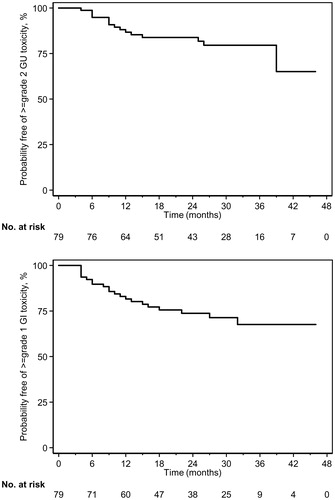
Late maximum GU toxicity was grade 0 (18%), 1 (62%), and 2 (20%). Late grade 2 GU toxicities consisted of urinary: frequency (11.4%), incontinence (6.3%), urgency (5.1%), hematuria (5.1%), retention (1.3%), and tract pain (1.3%). Median time to maximum late GU toxicity was 8 months (range 3–39). Kaplan-Meier survival estimate for late grade 2 GU toxicity-free survival is shown in . At 24 months, 83% were without late GU grade 2 toxicity. On univariate analysis baseline IPSS (OR 1.154, 95% CI: 1.048, 1.272, p = .004), baseline IPSS bother score (OR 1.539, 95% CI: 1.070, 2.213, p = .020), and concurrent ADT (OR 0.279, 95% CI: 0.089, 0.875, p = .029) were associated with late grade 2 GU toxicity; concurrent ADT (OR 0.247, 95% CI: 0.065, 0.942, p = .041 remained significantly associated on multivariate analysis, while baseline IPSS (OR 1.105, 95% CI: 0.988, 1.236, p = .081) and baseline IPSS bother (OR 1.360, 95% CI: 0.873, 2.117, p = .173) scores did not.
Figure 2. Kaplan-Meier survival estimates for late (A) GU grade 2 and (B) GI grade 1 toxicity–free survival for post-prostatectomy PT.
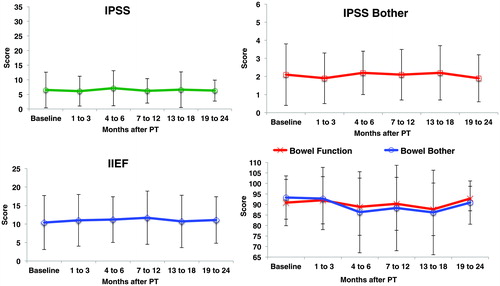
The mean (± standard deviation) patient reported outcome scores at baseline, 6 months, and 2 years were, respectively: IPSS 6.6 (±6.1), 7.1(±6.0), and 6.3 (±3.6); IPSS bother 2.1 (±1.7), 2.2 (±1.2), and 1.9 (±1.2); and IIEF 10.5 (±7.3), 11.2 (±6.2), and 11.1 (±6.3). Trends over time are shown in and Supplemental Table S1.
Figure 3. Patient reported outcomes (mean ± standard deviation) at baseline and after post-prostatectomy PT.
When assessing for an association between grade 2 GU toxicity and the bladder OAR DVH parameters on univariate analysis (Supplemental Table S2), only the following bladderless-CTV parameters were associated acutely: median dose (p = .040), V30 (p = .036), V40 (p = .031), and for late: maximum dose (p = .033).
GI toxicity
There were no grade ≥2 acute or late GI toxicities. Maximum acute GI toxicities were grade 0 (66%), 1 (34%) and consisted of diarrhea (14%), bloating (7%), abdominal pain (4%), hemorrhage (4%), rectal pain (3%), mucositis (2%), nausea (2%), and fecal incontinence (1%). Age (OR 0.940; 95% CI: 0.888, 0.995, p = .033) and combined modality PT and IMRT (OR 2.467; 95% CI: 1.023, 5.951, p = .044) were associated with acute grade 1 GI toxicity on univariate analysis, but not on multivariate analysis: age (OR 0.944, 95% CI: 0.891, 1.001, p = .052) and combined modality PT and IMRT (OR 2.289, 95% CI: 0.929, 5.637, p = .072)
Late maximum GI toxicities were respectively, grade 0 (73%) and 1 (27%), consisting mostly of mild diarrhea, hemorrhage, rectal pain, mucositis, and fecal incontinence. There were no univariate associations with late grade ≥1 GI toxicity and clinical characteristics (all Ps > .10). Median time to maximum late GI toxicity was 9 months (range 4–32). Kaplan-Meier survival estimates for late GI grade 1 toxicity-free survival is shown in . At 24 months, 74% were without late GI grade 1 toxicity. The mean (± standard deviation) patient reported outcome EPIC bowel scores ( and Supplemental Table S1) at baseline, 6 months, and 2 years were, respectively, for bowel function: 91 (±11), 89 (±14), and 93 (±6); and for bowel bother 93 (±15), 87 (±19), and 91 (±10).
Rectal parameters (Supplemental Table S3) associated with acute grade 1 GI toxicity were: rectal minimum dose (p = .009), median dose (p = .029), V10 (p = .015), V20 (p = .034), infield rectal minimum dose (p = .002), V10 (p = .007), V20 (p = .023), and ARW median dose (p = .022), V10 (p = .017), V20 (p = .016), V30 (p = .012), V40 (p = .012), V50 (p = .021), V60 (p = .044), and V65 (p = .044); late grade 1 GI toxicity was associated with infield rectal minimum dose (p = .041).
Discussion
In this study, we report for the first time—to the best of our knowledge—the clinical feasibility of post-prostatectomy PT and its associated toxicity profile. Acute and late maximum GU and GI toxicities were predominantly grade 0 and 1. Grade 2 GU toxicities were less common and no grade ≥3 toxicities were observed through a median 2-year follow up. Mean urinary, erectile, and bowel patient reported outcomes remained largely unchanged from baseline at 2 years despite some transient changes.
Comparisons to photon therapy
The toxicity rates noted compare favorably to previous toxicity rates reported for photon therapy. With respect to level I evidence, late grade ≥2 toxicity rates in randomized studies of adjuvant 2DRT historically ranged from 18 to 20% for GU toxicity and 2.5–3.3% for GI toxicity [Citation2, Citation3]. Direct comparisons of the toxicity rates in these historical studies are challenging given differences with current toxicity grading systems—for example, incontinence was not included in EORTC standard grading criteria. The largest retrospective post-prostatectomy IMRT series [Citation26] to date with 313 consecutive patients treated from 2004 to 2013 and median follow-up of 55 months reported GU grade ≥2 acute (25%) and late (74%) and GI grade ≥1 acute (72%) and late (30%) toxicities. Our previously reported institutional review of post-prostatectomy IMRT reported GU acute grade ≥2 toxicity of 9% (PB) and 22% (WP) [Citation9], comparable to the 15% in the present, mixed PT cohort. Late GU grade ≥2 toxicity was 23% overall in that IMRT study and 20% for this current study. GI acute grade ≥1 toxicity was 92% (WP) and 52% (PB), compared to the 34% in this cohort, while late GI grade ≥1 toxicity was 14% (WP) and 6% (PB) for IMRT and 27% for this study.
Toxicity associations
GU grade 2 acute toxicity was associated on multivariate analysis with only baseline IPSS bother score suggesting the importance of baseline bother in influencing tolerability of treatment. A previous analysis of acute toxicity in the first 100 prostate cancer patients undergoing IMRT with ERB similarly found that only baseline IPSS score was associated with acute GU toxicity on multivariate analysis [Citation17]. Only concurrent ADT was associated with late grade 2 GU toxicity. The association of ADT with late GU toxicity was previously reported in our post-prostatectomy IMRT institutional series [Citation9] and a pooled multi-institutional series [Citation26]. However, recently reported cooperative group randomized trials of salvage radiation with or without ADT did not find significant differences in acute or late GU effects [Citation7, Citation8].
While bladder DVH parameters have not consistently shown an association with GU toxicity in RT for prostate cancer [Citation27, Citation28], we did find univariate associations between intermediate dose level parameters and the bladderless-CTV acutely (): V30, V40, and median dose, and for late: maximum dose. An association with bladder V30 was previously reported in the largest single institution series to date of ∼1300 consecutive prostate cancer patients treated with PT in the radical setting [Citation29]. With median 5.3 years follow up, on multivariate analysis they found increasing percentages of grade ≥3 GU toxicity for patients with 0 (2%), 1 (4%), 2 (11%), or 3–4 (18%) these factors: bladder V30 > 19.2 cm3, median prostate volume >60 cc, pretreatment use of a-blockers, and pretreatment TURP.
Figure 4. Comparative mean dose volume histograms for bladder-related parameters and GU and rectal-related parameters and GI acute toxicities. The shaded regions (gray and pink) represent the standard deviations for each curve with the red shaded region representing the area of overlap between the 2 curves.
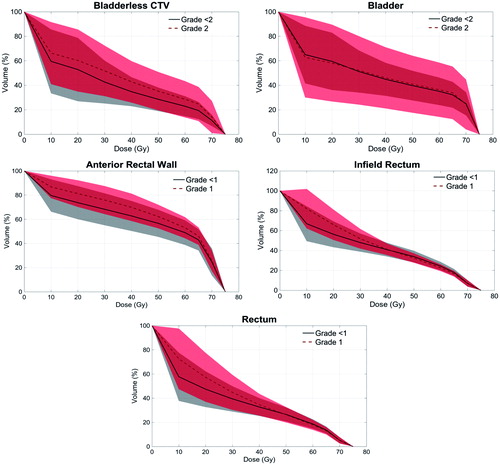
No clinical factors remained significantly associated with acute or late GI toxicity, although on multivariate analysis there was a marginal trend for age (p = .052) and combined modality treatment (PT + IMRT) (p = .072) and acute grade GI toxicity. More consistent dosimetric associations have been noted for rectal toxicity in radical treatment of intact prostate cancer, particularly in mid-high-dose regions [Citation30, Citation31]. Interestingly, acute grade 1 GI toxicity was associated with several low-intermediate rectal dose parameters including rectal minimum and median doses, V10 and V20, infield rectal minimum dose, V10, V20, and ARW median dose, V10 through V65 at all dose levels tested (). A report of over 1500 prostate cancer patients treated with PT in the intact setting found that all dosimetric parameters for the rectum and rectal wall were highly correlated with the risk of grade 2 rectal bleeding [Citation32], and break points best dichotomizing this risk were rectal V30 < 26.4% and V75 < 9.4% and rectal wall V30 < 27.7% and V75 < 9.2%. In our series of prostate cancer IMRT in the intact setting, infield rectum mean/median doses, V30, and V40 were similarly associated with GI grade 2 toxicity [Citation21].
The association of acute GU and GI toxicity with low and intermediate dose bladderless-CTV, ARW, and infield rectal DVH parameters in the setting of post-prostatectomy PT merits further investigation given the potential advantage of PT to reduce low and intermediate doses to these structures. Future treatment constraints may consider low to intermediate target dose levels in addition to the high dose targets more frequently used.
Patient reported outcomes trends
The mean baseline IPSS, IIEF, and EPIC bowel function and bowel bother scores showed mild transient changes, and by 2 years, the mean scores had largely unchanged from baseline values. These trends are similar to those reported for PT in the intact setting. A single institution report on 5-year clinical outcomes of 211 prostate cancer patients enrolled on 3 prospective PT trials found no significant changes in median pretreatment scores for bowel and urinary irritation, obstruction, and incontinence at greater than 4 years [Citation33]. Another single institution report on 226 men receiving PT for intact prostate cancer on a prospective, quality of life protocol with minimum 2 year follow up found that only EPIC bowel summary, function, and bother resulted in clinically meaningful decrements beyond treatment completion and through 24-month follow-up; cumulative grade ≥2 GU and GI toxicity at 24 months were 13% and 10%, respectively [Citation34].
Limitations of this study include the retrospective nature and heterogeneous cohort composed of patients treated with or without IMRT and/or ADT and/or elective pelvic nodal irradiation. Still, we chose to assess this entire heterogeneous cohort as we felt that it represented a more realistic sample of the variety of post-prostatectomy cases presenting for RT with pursuit of PT from the outset. Additional limitations of the study include the limited number of patients and follow up. It is possible that bladder and rectal function could decline with time, and continued follow-up could reveal additional GU and GI toxicities and significant associations. Despite these limitations, we felt that an initial report was merited since the feasibility and toxicity profile of post-prostatectomy PT had not yet been reported and it may guide the evolution of ongoing therapy and further allay toxicity concerns deterring referral.
In conclusion, post-prostatectomy PT for prostate cancer is clinically feasible with a favorable GU and GI toxicity profile comparable to photon therapy acutely and through early follow up in the first 100 patients undergoing adjuvant or salvage therapy at our institution.
Supplemental Material
Download Zip (2.9 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol. 2013;190(2):441–449.
- Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 2012;380(9858):2018–2027.
- Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181(3):956–962.
- Wiegel T, Bartkowiak D, Bottke D, et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol. 2014;66(2):243–250.
- Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–2041. Erratum in: J Clin Oncol. 2007;25(26):4153.
- Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 2008;299(23):2760–2769.
- Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376(5):417–428.
- Carrie C, Hasbini A, de Laroche G, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2016;17(6):747–756.
- Deville C, Vapiwala N, Hwang WT, et al. Comparative toxicity and dosimetric profile of whole-pelvis versus prostate bed-only intensity-modulated radiation therapy after prostatectomy. Int J Radiat Oncol Biol Phys. 2012;82(4):1389–1396.
- Weiner AB, Matulewicz RS, Schaeffer EM, et al. Contemporary management of men with high-risk localized prostate cancer in the United States. Prostate Cancer Prostatic Dis. 2017;20(3):283–288.
- Fontenot JD, Lee AK, Newhauser WD. Risk of secondary malignant neoplasms from proton therapy and intensity-modulated x-ray therapy for early-stage prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74(2):616–622.
- Slater JD, Rossi CJ Jr, Yonemoto LT, et al. Proton therapy for prostate cancer: the initial Loma Linda University experience. Int J Radiat Oncol Biol Phys. 2004;59(2):348–352.
- Talcott JA, Rossi C, Shipley WU, et al. Patient-reported long-term outcomes after conventional and high-dose combined proton and photon radiation for early prostate cancer. JAMA 2010;303(11):1046–1053.
- Trofimov A, Nguyen PL, Coen JJ, et al. Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: a treatment planning comparison. Int J Radiat Oncol Biol Phys. 2007;69(2):444–453.
- Weber DC, Zilli T, Vallee JP, et al. Intensity modulated proton and photon therapy for early prostate cancer with or without transperineal injection of a polyethylen glycol spacer: a treatment planning comparison study. Int J Radiat Oncol Biol Phys. 2012;84(3):e311–e318.
- Swisher-McClure S, Yin L, Rosen M, et al. Prospective MRI-based imaging study to assess feasibility of proton therapy for post-prostatectomy radiation. Acta Oncol. 2016;55(7):828–833.
- National Cancer Institute Common TerminologyCriteria for Adverse Events v4.0. NCI, NIH, DHHS. 29 May 2009. NIH publication # 09-7473.
- Barry MJ, Coffey DC, Fitzpatrick J, et al. Recommendations of the International Consensus Committee concerning patient evaluation for research studies. In: Cockett ATK, Aso Y, Chatelain C, et al., editors. Proceedings of the International Consultation on Benign Prostatic Hyperplasia (BPH). Channel Islands: Paris Scientific Communication International; 1991. p. 279–281.
- Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11(6):319–326.
- Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 2000;56(6):899–905.
- Deville C, Both S, Bui V, et al. Acute gastrointestinal and genitourinary toxicity of image-guided intensity modulated radiation therapy for prostate cancer using a daily water-filled endorectal balloon. Radiat Oncol. 2012;7:76.
- Michalski JM, Lawton C, El Naqa I, et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;76(2):361–368.
- Lawton CA, Michalski J, El-Naqa I, et al. RTOG GU Radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74(2):383–387.
- Kirk ML, Tang S, Zhai H, et al. Comparison of prostate proton treatment planning technique, interfraction robustness, and analysis of single-field treatment feasibility. Pract Radiat Oncol. 2015;5(2):99–105.
- International Commission on Radiation Units and Measurements.Prescribing, recording, and reporting proton-beam therapy ICRU Report 78. Oxford: Oxford University Press; 2007.
- Jensen L, Yuh B, Wong JYC, et al. Outcomes and toxicity of 313 prostate cancer patients receiving helical tomotherapy after radical prostatectomy. Adv Radiat Oncol. 2017;2(4):597–607.
- Cheung MR, Tucker SL, Dong L, et al. Investigation of bladder dose and volume factors influencing late urinary toxicity after external beam radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2007;67(4):1059–1065.
- Peeters ST, Hoogeman MS, Heemsbergen WD, et al. Volume and hormonal effects for acute side effects of rectum and bladder during conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2005;63(4):1142–1152.
- Bryant C, Smith TL, Henderson RH, et al. Five-year biochemical results, toxicity, and patient-reported quality of life after delivery of dose-escalated image guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2016;95(1):422–434.
- Heemsbergen WD, Hoogeman MS, Hart GA, et al. Gastrointestinal toxicity and its relation to dose distributions in the anorectal region of prostate cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61(4):1011–1018.
- Fiorino C, Sanguineti G, Cozzarini C, et al. Rectal dose-volume constraints in high-dose radiotherapy of localized prostate cancer. Int J Radiat Oncol Biol Phys. 2003;57(4):953–962.
- Colaco RJ, Hoppe BS, Flampouri S, et al. Rectal toxicity after proton therapy for prostate cancer: an analysis of outcomes of prospective studies conducted at the university of Florida Proton Therapy Institute. Int J Radiat Oncol Biol Phys. 2015;91(1):172–181.
- Mendenhall NP, Hoppe BS, Nichols RC, et al. Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2014;88(3):596–602.
- Pugh TJ, Munsell MF, Choi S, et al. Quality of life and toxicity from passively scattered and spot-scanning proton beam therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2013;87(5):946–953.
