Abstract
Background: There is paucity of evidence regarding the optimal follow-up (FU) regimen for lung cancer. Consequently, FU is organized differently across countries. The Danish FU regimen has short FU intervals with a computed tomography (CT) scan of the chest and upper abdomen every three months in the early phase (first 2 years), then every six months in the late phase of FU (3rd, 5th year). Characterizing recurrences missed by the FU program in terms of site, tumor histology, department, and phase of FU, could improve the FU program.
Material and method: A case–control study of curatively treated stage I lung cancer patients who attended the Danish FU-program and had recurrence identified through the follow-up program (controls, FU group) or outside FU program (cases, symptomatic group).
Results: Of 233 included patients with recurrence, the FU group constituted 85% (n = 197). Among the 15% (n = 36) in the symptomatic group, 53% had involvement of the central nervous system compared with 3% in the FU group. The unadjusted odds ratio (OR) for having an isolated brain recurrence (IBR) in the symptomatic group was 52.3 (95%CI: 15.1–181.4) as compared with the FU group. The OR for having a symptomatic recurrence in the early phase of FU was 2.5 (95%CI: 0.7–8.7) compared with the late phase.
Conclusions: The FU program did not identify the majority of patients with IBR. Including cerebral imaging in the FU program may result in an earlier detection of brain metastases. These matters should be studied in a prospective setting.
Introduction
A larger proportion of patients with lung cancer receive treatment with curative intent [Citation1]. Follow-up (FU) of these patients is generally recommended [Citation2] in order to identify recurrence as early as possible to increase survival although, the evidence supporting these recommendations is weak [Citation3]. This allows for differences in clinical practice between different countries and health care providers [Citation4], more so than for patients in surveillance following palliative oncological treatment [Citation5]. It is well established that the rate of recurrence is high even among early stage lung cancer patients [Citation6,Citation7]. Guidelines currently recommend local treatment (primarily surgery or stereotactic body radiotherapy) for stage I lung cancer. Systemic adjuvant therapy is only recommended for a subgroup of these patients [Citation8]. Thus, recurrence in these patients is due to either failed local treatment, and/or the presence of undetected (micro) metastases at completion of treatment.
According to the Danish FU scheme, a lung cancer patient who has undergone curative treatment will be offered a contrast-enhanced CT of the chest and the upper abdomen and a clinical evaluation every 3 months for the first 2 years (early phase), then every 6 months for an additional 3 years (late phase). Depending on treatment modality and health care region, responsibility for FU in Denmark lies either with departments of pulmonology or oncology, but complying to the same national FU guidelines which have been in effect since 2011 [Citation9]. To our knowledge, no studies have assessed whether the quality of the FU-program depends on which department is conducting the FU.
There is a scarcity of knowledge both regarding the fraction of recurrences identified through the intensive FU conducted in Denmark [Citation4], but also what characterizes those patients with recurrence who are not identified through the FU program. In relation to a comprehensive review of medical records, we explored if any differences occur between recurrences detected within or outside the FU in terms of clinical characteristics, site of recurrence, and phase of FU. Our findings can point to potential improvements of the Danish FU program.
Method
This is a case–control study of Danish stage I lung cancer patients diagnosed with recurrent disease after completing treatment with curative intent.
Data sources and study population
We retrospectively identified the study population for the present recurrence study from two unpublished population-based matched case–control studies, where we had detailed information on the FU from medical records. All patients had been diagnosed from 1 January 2011 until 31 December 2014 and were registered with clinical stage I lung cancer (TNM 7th edition) [Citation10] in the Danish Lung Cancer Register (DLCR) [Citation11].
Eligible for inclusion into the present study, were patients who completed curative therapy, attended the FU program and subsequently had recurrence of lung cancer. Thus, we excluded patients who were either not treated or did not complete curative treatment, patients who died prior to the first FU visit, patients not attending the FU program, patients considered to have a new lung cancer, and patients with missing data regarding recurrence ().
Figure 1. Flowchart of the process leading to the identification of the study population of stage I lung cancer patients with recurrence.
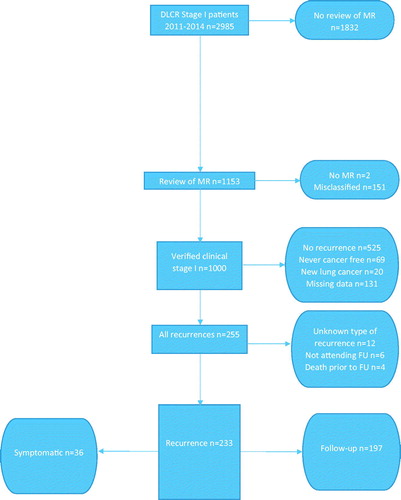
We defined patients as cases if they were diagnosed with recurrence based on symptoms leading to admission or additional investigations outside the FU program, denoted symptomatic recurrence (symptomatic group). For the remaining part of the study population, recurrence was diagnosed in relation to a FU visit (controls, FU group). This was based either on the imaging results or on the clinical evaluation, including self-reported symptoms.
Study variables
The main study variable was site of recurrence, based on the results of the diagnostic work-up for recurrence. The length of the FU interval is dependent on phase of follow-up and we established in which year of FU the recurrence was diagnosed. The department conducting the FU was assessed for the two groups.
Statistical analysis
Proportional distribution, presented with two-sided p values, was used to describe differences between the symptomatic and the FU group in terms of baseline characteristic and differences in distributions of site of recurrence according to phase of FU.
A logistic regression model was used to investigate the difference between the symptomatic and FU groups. The variables site and phase of FU at time of recurrence (early/late) were included as categorical variables in the model. Calculations were performed with SAS software (SAS system, SAS Institute, Cary, NC, USA) and Stata Software (StataCorp 4905 Lakeway Drive College Station, TX, USA).
Results
As seen in , we identified 255 recurrences among 1000 eligible patients. We excluded 22 patients with recurrence, due to either missing data regarding FU, non-participation in the FU program or death prior to the onset of the FU program. Of the remaining 233 recurrences, 15% had a symptomatic recurrence (n = 36) and 85% were identified via the FU program (n = 197).
Baseline parameters are presented in . As seen, there was a similar age, gender and treatment distribution in the symptomatic and the FU group. Furthermore, the majority of patients in both groups were followed in the oncology department.
Table 1. Descriptive characteristics of stage I lung cancer patients with recurrence, according to whether recurrence was diagnosed due to symptoms or in relation to the FU-program.
Site of recurrence
As seen in , distant recurrence (including CNS recurrence) was the most common type in both groups. However, there was a significant difference, in terms of locoregional recurrence, which occurred more frequently in the FU group, while CNS recurrence was more frequent in the symptomatic group.
Brain metastases
Among the total 1000 verified clinical stage I patients that we reviewed (), 24 (2.4%) had recurrences involving the CNS.
In the symptomatic group, 17 (47%) had isolated CNS recurrence and two with a combined recurrence had CNS involvement, thus 53% of the recurrences in the symptomatic group had CNS involvement. In the FU group, only four (2%) had isolated CNS recurrence and one (0.5%) with a combined recurrence had CNS involvement.
To focus on patients who could potentially be diagnosed with isolated brain recurrence in the follow-up period (IBR), we excluded all patients with a combined recurrence (n = 41) and one patient with a spinal metastasis. Sixteen of the remaining 29 patients in the symptomatic group and four of the remaining 162 patients in the FU group had IBR. Consequently, the unadjusted OR of being diagnosed with IBR among patients with symptomatic recurrence was 52.3 (95% CI: 15.1–181.4) compared with the FU group (result not shown).
We did not categorize the symptoms leading to the symptomatic isolated brain recurrence, but they included epileptic seizures and other cramps, headaches, and stroke-like symptoms. Of the 16 patients with IBR in the symptomatic group, seven (44%) were treated with curative intent (surgery 3, radiotherapy 4), the remaining nine were treated palliatively (incl. supportive care only). Among the four with IBR in the FU group, two were treated with curative intent and two were treated palliatively.
Of the 24 patients with recurrence involving the CNS, 70% had an adenocarcinoma and 8% had a squamous cell carcinoma. After excluding subjects with CNS-recurrence from the logistic regression analysis, there was no difference in site of recurrence between the two groups (data not shown).
Recurrence in the early versus the late phase of follow-up
We tested if first year and second year of FU could be combined when analyzing the association between phase of FU and site of recurrence and found no difference (p value = .40). As seen in , the majority of all recurrences (190/233, 82%) occurred in the early phase period of the FU where most of the local recurrences as well as nodal recurrences were diagnosed. In spite of the short FU intervals, the OR of experiencing a symptomatic recurrence in the early phase was 2.5 (95%CI: 0.7–8.7) compared with in the late phase.
Table 2. Sites of recurrence among stage I lung cancer patients diagnosed with recurrence in early phase (0–2 years) and late phase (2–5 years) of follow-up.
Discussion
The current study evaluated the Danish FU program in a population-based setting. This area of clinical practice has limited consensus [Citation12–14]. The multi-center randomized phase III study of post-surgical non-small cell lung cancer follow-up, comparing FU with chest X ray and CT scan by Westeel et al. [Citation15] have so far not proven a significant survival benefit of a CT-based FU-regimen. However, a caveat of assessing the long-term outcome of a given FU-program prospectively is that more recent advances in treatment might not be reflected. A single-center Japanese retrospective study by Shimada et al. [Citation16] included 917 surgically treated stage I lung cancer patients with available FU information assessed the impact of several clinicopathological variables. They found that receiving any kind of post-recurrence treatment was associated with an improved outcome, but the authors correctly identified the potential selection bias when assessing clinical interventions in risk estimates. Thus, it remains challenging to assess the effect of a part of clinical practice as complex and of such long duration as the FU program for lung cancer. However, in contrast to most patient related risk factors, elements of the FU program can be adapted as we learn more.
In our study population, 15% of the disease recurrences were not identified in relation to the FU program, and the majority of these patients had distant recurrence. On the other hand, the vast majority of our study subjects with locoregional recurrence were identified in relation to the FU program. There was no difference between the symptomatic and the FU group in terms of follow-up department.
The OR for having a symptomatic recurrence in the early phase was 2.5 as compared with the late phase, however, not statistically significant, this finding suggests that the 3 month-FU intervals may be justified as the main purpose of the systematic FU with CT is to identify recurrences before they become symptomatic.
The proportion of brain metastases was 2.4% in our data set of 1000 patients with stage I lung cancer. In spite of a very intensive FU regime as the Danish, patients with IBR were mainly diagnosed due to symptoms resulting in hospital admission or GP consultation and not in relation to the FU program. Cerebral imaging () was infrequently used in the FU and it appeared that the clinical evaluation was insufficient at identifying patients with IBR. This is supported by a small study (n = 91) by Hochstenbag et al., who found a low sensitivity of a clinical evaluation by a neurologist in identifying brain metastases [Citation17]. Since we only identified patients with symptomatic brain metastases and not those with asymptomatic, the actual proportion could arguably be higher than what we found.
In terms of histology, the majority of IBR patients had an adenocarcinoma, which is in accordance with the recent findings in a systematic review of NSCLC patients with brain metastases by Fenske et al. [Citation18], and a retrospective study of risk factors for brain recurrence by Consonni et al. [Citation19]. There are strong suggestions that an improved survival may be achieved with an early identification of recurrence for patients with IBR [Citation20] where effective treatment modalities exist for cerebral metastases.
The effect of additional examinations (including a cerebral CT) in the FU of all stages of lung cancer has been studied by Gourcerol et al. [Citation21] in a French single-center surgical cohort study of 162 patients (90% male patients) included from 1990 to 2002. However, no conclusions could be made regarding the particular effect for the cerebral CT since the compliance relating to this procedure was only 24%. Nevertheless, in their study, 52% were asymptomatic at time of recurrence and these patients were found to have longer survival (not only due to lead time bias) and received curative therapy to a larger extent than patients with symptomatic recurrence. However, in our study a patient could be symptomatic and belong to the FU group, so these numbers cannot be directly compared, but they still indicate a favorable outcome relating to having recurrence diagnosed before the development of symptoms. The implementation of radical therapy against metastatic brain tumors in surgically treated NSCLC patients (n = 40) was studied by Kanou et al. It was found that receiving treatment when the tumor was still small and asymptomatic, contributed to an increase in patients' life expectancy with a 22.5% 5-year survival rate from the diagnosis of brain recurrence [Citation22].
By incorporating cerebral imaging into the algorithm, the FU of lung cancer patients may be improved in terms of identifying a larger proportion of patients via the FU program prior to the development of symptoms. The optimal imaging modality and the impact in terms of overall survival and number needed to treat need to be studied further. Since we have only looked upon stage I patients, with an a priori low risk of cerebral recurrence, one could expect that the potential benefits of adding cerebral imaging would be even greater in the FU of patients with more advanced disease with increased risk of developing brain metastases [Citation23,Citation24].
Strengths and limitations
Our comprehensive review of medical records and the detailed information we obtained regarding the FU phase provided valuable insight into areas of the follow-up regimen, which has not formerly been studied in our population. We have included patients from all departments of pulmonology and oncology, and have thus avoided center-specific observation and selection bias. Our study has, however, several limitations. We retrieved the information regarding the recurrences from the medical records thus allowing for interobserver bias, since different definitions of recurrence versus metachronous lung cancer could have been used at different hospitals. All cerebral recurrences were verified radiologically, none bioptically, and there is potential for a differential diagnosis mimicking cerebral metastasis. Only a minority of the patients with IBR was subjected to extra-cerebral diagnostic work-up to systematically assess additional disease burden.
Conclusions
The Danish FU program was not able to identify the vast majority of patients with isolated cerebral recurrence in our population of curatively treated stage I lung cancer patients, where the occurrence of CNS recurrence was 2.4% and primarily among patients with an adenocarcinoma. On the other hand, the majority of patients with locoregional recurrence were identified within the FU-program. Incorporating cerebral imaging into the FU program, might improve this. However, the potential benefit of such practice should be evaluated prospectively on a non-selected study population.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Jakobsen E, Rasmussen TR, Green A. Mortality and survival of lung cancer in Denmark: results from the Danish Lung Cancer Group 2000–2012. Acta Oncol (Madr). 2016;55:2–8. DOI:10.3109/0284186X.2016.1150608.
- Colt HG, Murgu SD, Korst RJ, et al. Follow-up and surveillance of the patient with diagnosis and management of lung cancer, 3rd ed: American College of. Chest Physicians. Chest. 2013;143:e437S–e454S. DOI:10.1378/chest.12-2365.
- Helsedirektoratet. Nasjonalt Handlingsprogram med Retningslinjer for Diagnostikk, Behandling og Oppfølging av Lungekreft, Mesoteliom og Thymom [Internet]. Norway, Oslo, Helsedirektoratet. 2016 June. Norwegian. Available from: https://helsedirektoratet.no/r.
- Christensen NL, Jekunen A, Heinonen S, et al. Lung cancer guidelines in Sweden, Denmark, Norway and Finland: a comparison. Acta Oncol (Madr). 2017;56:943–948. DOI:10.1080/0284186X.2017.1315172.
- Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:V1–V27. DOI:10.1093/annonc/mdw326.
- Robinson CG, Dewees TA, Naqa IME, et al. Patterns of failure after stereotactic body radiation therapy or Lobar Resection for. JTO Acquis. 2013;8:192–201. DOI:10.1097/JTO.0b013e31827ce361.
- Fedor D, Johnson WR, Singhal S. Local recurrence following lung cancer surgery: incidence, risk factors, and outcomes. Surg Oncol. 2013;22:156–161. DOI:10.1016/j.suronc.2013.04.002.
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv1–iv21. DOI:10.1093/annonc/mdx222.
- Sundhedsstyrrelsen. Kraeftpakkeforløb For Lungecancer [Internet]. Denmark, Copenhagen, Sundhedsstyrelsen; 2012 [Updated 2013 Jul 1]. Danish. Available From: http://sundhedsstyrelsen.dk/∼/media/30309E0576AF42CF95138AC3CE59CF74.ashx.
- Mirsadraee S. The 7th lung cancer TNM classification and staging system: review of the changes and implications. Wjr. 2012;4:0. DOI:10.4329/wjr.v4.i4.128.
- Jakobsen E, Green A, Oesterlind K, et al. Nationwide quality improvement in lung cancer care: the role of the Danish Lung Cancer Group and Registry. J Thorac Oncol. 2013;8:1238–1247.
- Calman L, Beaver K, Hind D, et al. Survival benefits from follow-up of patients with lung cancer: a systematic review and meta-analysis. J Thorac Oncol. 2011;6:1993–2004.
- Ho C, Siegfried J, Remo K, et al. Adherence to surveillance guidelines in resected NSCLC: physician compliance and impact on outcomes. Lung Cancer. 2017;112:176–180. (June 2016):DOI:10.1016/j.lungcan.2017.08.012.
- Schmidt-Hansen M, Baldwin DR, Hasler E. What is the most effective follow-up model for lung cancer patients? A systematic review. J Thorac Oncol. 2012;7:821–824. DOI:10.1097/JTO.0b013e31824afc55.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier INCT00198341, Post-Surgical Non-Small Cell Lung Cancer (NSCLC) Follow-up. Available from: https://clinicaltrials.gov/ct2/show/NCT00198341?term=IFCT.
- Shimada Y, Saji H, Yoshida K, et al. Prognostic factors and the significance of treatment after recurrence in completely resected stage I non-small cell lung cancer. Chest. 2013;143:1626–1634. DOI:10.1378/chest.12-1717.
- Hochstenbag MMH, Twijnstra A, Hofman P, et al. MR-imaging of the brain of neurologic asymptomatic patients with large cell or adenocarcinoma of the lung. Does it influence prognosis and treatment? Lung Cancer. 2003;42:189–193. DOI:10.1016/S0169-5002(03)00291-5.
- Fenske DC, Price GL, Hess LM, et al. Systematic review of brain metastases in patients with non-small-cell lung cancer in the United States, European Union, and Japan. Clin Lung Cancer. 2017;18:607–614. DOI:10.1016/j.cllc.2017.04.011.
- Consonni D, Pierobon M, Gail MH, et al. Lung cancer prognosis before and after recurrence in a population-based setting. JNCI J Natl Cancer Inst. 2015;107:djv059. DOI:10.1093/jnci/djv059.
- Niibe Y, Nishimura T, Inoue T, et al. Oligo-recurrence predicts favorable prognosis of brain-only oligometastases in patients with non-small cell lung cancer treated with stereotactic radiosurgery or stereotactic radiotherapy: a multi-institutional study of 61 subjects. BMC Cancer. 2016;16:659. DOI:10.1186/s12885-016-2680-8.
- Gourcerol D, Scherpereel A, Debeugny S, et al. Relevance of an extensive follow-up after surgery for nonsmall cell lung cancer. Eur Respir J. 2013;42:1357–1364. DOI:10.1183/09031936.00086712.
- Kanou T, Okami J, Tokunaga T, et al. Prognostic factors in patients with postoperative brain recurrence from completely resected non-small cell lung cancer. Thorac Cancer. 2015;6:38–42. DOI:10.1111/1759-7714.12137.
- Dawe DE, Greenspoon JN, Ellis PM. Brain metastases in non-small-cell lung cancer. Clin Lung Cancer. 2014;15:249–257. DOI:10.1016/j.cllc.2014.04.008.
- Germain F, Wai ES, Berthelet E, et al. Brain metastasis is an early manifestation of distant failure in stage III nonsmall cell lung cancer patients treated with radical chemoradiation therapy. Am J Clin Oncol. 2008;31:561–566. DOI:10.1097/COC.0b013e318172d5f9.
