Abstract
Aim: Several trials have shown that preoperative (chemo)radiotherapy (CRT) reduces local recurrence rates (LRRs) in rectal cancer (RC). The use of CRT varies greatly between countries. It is unknown whether the restrictive use of CRT in Denmark results in a higher LRR relative to other countries. The aim was to evaluate the LRR in a national Danish consecutive cohort of patients with RC.
Methods: All data from patients with RC in Denmark in 2009–2010 who were operated on with curative intent were retrieved from the Danish Colorectal Cancer Group database. Patients with metastases at the time of diagnosis, patients with synchronous colon cancer, and patients, in whom only local surgical procedures were performed, were excluded. In total, 1633 patients met the inclusion criteria. Clinical follow-up was at least five years with a cut-off date of 31 December 2015.
Results: Clinical follow-up was 5.4 years (median) with an interquartile range of 4.5–6.1 years. Of all included patients, 479 (29%) were treated with preoperative long-course CRT. Local recurrence was found in 68 patients, resulting in an LRR of 4.2%, and 182 (11%) patients developed distant metastases. Five-year overall survival was 74% (95% CI: 71.64–75.91).
Conclusions: Five-year follow-up of curatively treated patients with RC in Denmark revealed a low LRR. This figure is identical to those reported in other Nordic countries, despite Denmark’s considerably stricter guidelines for CRT. The obtained results justify the currently adopted restrictive use of preoperative CRT in Denmark.
Background
Rectal cancer (RC) has a relatively high annual incidence of 15–25/100,000 in Europe and the United States [Citation1,Citation2]. Improved staging, surgical procedures, radiotherapy, chemotherapy, and biological agents have all contributed to the current five-year cancer specific survival rate of approximately 60% [Citation3].
Locally advanced RC (LARC) is a challenging clinical situation in which the treatment aims are to minimize the risk of local recurrence, which is a feared outcome. In randomized trials, preoperative radiotherapy has been consistently proven to be effective at reducing local recurrence rates (LRRs) [Citation4,Citation5]. However, in earlier randomized trials, reduced LRRs have not led to a prolongation of overall survival (OS) [Citation6]. Preoperative chemoradiotherapy (CRT) has significantly increased the rate of late morbidity compared to surgery alone as reported in both a randomized trial and in registry studies [Citation7–9]. In a Nordic population-based registry study from the 1990s, LRRs were reported to be high (9–23%) after curatively intended surgery [Citation10]. In this publication, the LRR for the Danish cohort could not be reported due to missing register data. LRRs have diminished significantly following the introduction and establishment of total mesorectal excision (TME) by Heald and colleagues [Citation11]. Numerous subsequent publications of randomized trials and registry studies cite LRRs between 4.4% and 8.0% [Citation5,Citation6, Citation12–14].
According to the Danish Colorectal Cancer Group (DCCG.dk) [Citation15], approximately 45% of the Danish patients with RC have tumors that are resectable at the time of diagnosis and are primarily treated with surgical procedures alone. Twenty percent of the patients have locally advanced resectable tumors and obtain preoperative CRT. Twenty percent of the patients are diagnosed with stage IV disease and are treated with chemotherapy and/or biological agents. Fifteen percent of the patients have an unknown stage of the disease; they are often patients of advanced age, patients who reject surgical procedures or patients with a severe comorbidity for whom full clinical staging has no consequence.
The number of Danish patients treated with CRT differs significantly from the number of patients treated with CRT in other countries, representing approximately 30% of resected RC patients in Denmark. In other European countries, this number ranges between 40% and 80% [Citation16]. A Norwegian publication reports the use of preoperative CRT in almost 40% of the patients [Citation17], and a Swedish publication reports that 66% of the patients are preoperatively treated with CRT during the years of the current study [Citation18]. Danish guidelines are rather strict in the selection of patients for preoperative CRT (see Material and methods) [Citation19]. These guidelines are intended to prevent late morbidity due to bowel obstruction, sexual dysfunction, and fecal incontinence in as many patients as possible [Citation8,Citation9,Citation20,Citation21]. It is unknown whether this restrictive treatment strategy results in a higher LRR in Denmark than in internationally published data.
Recently, a study compared the outcomes of patients with RC in Norway and Sweden in terms of LRR and OS [Citation22]. The two countries have different treatment algorithms with great differences in the percentage of patients receiving preoperative CRT. The authors reported a difference in outcomes in the 1990s, but recently, no difference in LRRs could be detected.
The aim of the study was to investigate the LRR in a national Danish consecutive cohort of resected RC patients.
Material and methods
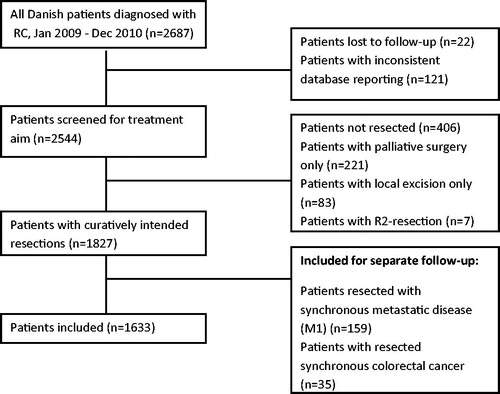
RC management in Denmark was carried out at 18 surgical centers and 7 oncology centers. All newly diagnosed patients with RC (International Code of Disease, 20.9) histopathologically defined as adenocarcinoma were recorded in the DCCG.dk database. This nation-wide database mainly includes demographics, which are supplemented by perioperative surgical data and histopathological, radiological, and oncological variables. The database had a completeness of more than 98% during the study period [Citation23]. All registry information from this consecutive nation-wide cohort diagnosed from 1 January 2009 to 31 December 2010 was retrieved and stored in the study database (n = 2687). In the first data export, several variables, including histopathology, were missing. The registry was updated by asking the corresponding pathology departments to complete the registry data for approximately 500 patients. After this update, a new data extraction step was performed. The study period was chosen because treatment-related guidelines were implemented during the three years before the study and had not changed afterwards, and because the clinical follow-up data of all patient files had to be manually reviewed. All patients who had surgery with curative intent were selected for further analysis (n = 1930). Patients with metastatic disease at diagnosis (n = 164) and patients with synchronous colon cancer (CC) (n = 35) were followed up separately. Patients who had only local surgery (e.g., transanal endoscopic microsurgery) were excluded (n = 82) unless histopathological examination resulted in immediate full surgical resection. Finally, patients with an R2-resection (n = 5) were also excluded. R2 was defined as a macroscopically residual tumor visible in the surgical field as judged by the surgeon at the end of the procedure. In all, 1633 patients were included in this study, as shown in .
Figure 1. Flowchart showing all patients diagnosed with RC. RC, rectal cancer. R2, macroscopic non-radical resection.
The diagnostic workup comprised a complete colonoscopy, a biopsy of the suspected tumor, rigid proctoscopy to measure tumor height from the anal verge (low 0–5 cm, mid 5–10 cm and high 10–15 cm), MRI of the lower abdomen and pelvis, and a CT scan of the thorax/abdomen to assess the Tumour Node Metastasis (TNM) stage. Based on diagnostic staging, patients were (a) referred to surgery without preoperative treatment or (b) referred to preoperative CRT in cases of T3/T4 (a and b) tumors in low RC, all T3/T4 (a and b) tumors in mid RC with a distance to the mesorectal fascia of less than 5 mm measured by MRI (mrMRF) or non-resectable T4 (b) tumors in high RC. CRT consisted of 50.4 Gy/28 fractions with concomitant capecitabine 850 mg/m2 BID. In cases with patients of advanced age or with severe comorbidities, concomitant capecitabine might have been omitted or radiotherapy was given as 25 Gy in five fractions. Surgery was performed according to national guidelines [Citation24]. In cases of preoperative CRT, re-staging and surgery was performed after 6–8 weeks. During the project period, patients were referred to postoperative adjuvant chemotherapy given as 5-Flurouracil (± oxaliplatin) in cases in which histopathology demonstrated high-risk stage II or stage III disease with no considerations of whether the patients had preoperative treatment or not [Citation25]. Postoperative radiotherapy was not recommended.
During the study period, no standardized follow-up program existed in Denmark. However, most patients were seen regularly at surgical departments with clinical examinations and endoscopic procedures. Furthermore, a CT scan of the thorax/abdomen/pelvis was performed after one and three years. Some patients refused follow-up, and for some patients, the responsible physician decided to end the follow-up, most often due to the patient’s advanced age or cognitive dysfunction.
All clinical follow-up data were obtained from patients’ surgical, oncological, and medical files for at least five years. The cut-off date was 31 December 2015. In case of outpatient visits to departments other than the department of surgery, the date of this visit was used for follow-up.
Local (or loco-regional) recurrence was defined as any recurrent tumor growth in the pelvic cavity as documented by clinical, radiological, or histopathological examination.
Distant recurrence was defined as any tumor growth distant to the pelvic region mentioned above as documented by clinical, radiological, or histopathological examination.
During the follow-up period, all subsequent primary events were recorded: (1) local recurrence, 92) distant metastases, or (3) death.
Patients with synchronous CC, patients with metastatic disease at diagnosis, and patients with distant recurrences as a first event were all included for separate follow-up to determine whether they presented with local recurrence as a later event during the follow-up period.
The primary endpoint of the investigation was to obtain the LRR in the cohort of resected RC patients. Secondary endpoints were their distant recurrence rate and OS.
This study was approved by the National Data Protection Agency (2008-58-0028) and the Danish Patient Safety Authority (3-3013-1580/1/).
Statistics
All data extracted from the national DCCG.dk database and supplementary data from the patients’ files were transferred to a research database designed specifically for this study. Statistical analyses were performed with the statistical software Stata, version 15 (StataCorp, College Station, Texas, USA). Demographic data together with tumor and treatment characteristics were described by a frequency table. P values were calculated by a paired t-test for age and Fisher’s exact test for the remaining variables in . LRRs and distant recurrence rates were calculated by using the cumulative incidence accounting for competing risk of death by means of the Aalen–Johansen estimator [Citation26]. OS was estimated using Kaplan-Meier plots. A Cox proportional hazards regression model was used to model the impact on local and distant recurrence from (y)pTNM, R-status and preoperative CRT, calculating both crude and adjusted hazard ratios, where adjusted hazard ratios were mutually adjusted for each other. Due to the limited number of events, only the three variables mentioned previously were used in the regression model.
Table 1. Characteristics of the 1633 included patients.
Results
The characteristics of the 1633 included patients are shown in . A total of 479 patients were treated with CRT and 1092 patients were not treated with CRT. For 62 patients, no information regarding preoperative treatment was found. Data on diagnostic MRI variables as well as use of adjuvant chemotherapy were not available. The clinical follow-up was 5.4 years (median) with an interquartile range of 4.5–6.1 years.
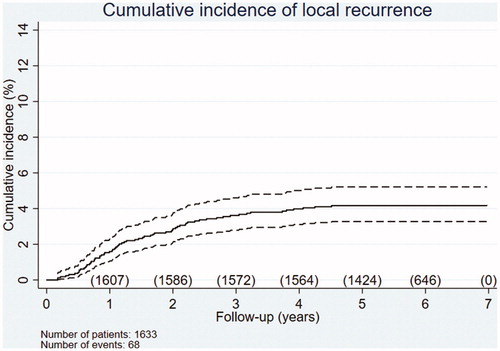
Of the 1633 included patients with RC, 44 developed local recurrence as a first event (2.7%) and 24 developed local recurrence synchronously with distant recurrence; see below. Thus, in total, local recurrences were found in 68 patients, corresponding to an LRR of 4.2%. shows the cumulative incidence of local recurrence over time.
Figure 2. Cumulative incidence of local recurrence during the follow-up period. The number of patients at risk is shown per year. Of the 1633 patients with RC, 68 (LRR 4.2%) developed local recurrence. Dotted lines represent 95% confidence intervals.
Among the 68 patients with local recurrence, baseline characteristics (Supplementary Table A) showed that 22 patients had high RC, 21 had mid RC, and 23 had low RC as their primary tumor location. In two patients, tumor height was not found in the registry. Preoperative CRT was performed in 19 patients and no CRT was given in 40 patients. Regarding residual tumor (R-status), 8 patients had a R1-resection and 48 had a R0-resection. A total of 12 patients had no recorded information regarding R-status. The T-category showed that 13/68 patients had only (y)pT2-tumors and in the N-category, 32/68 with (y)N0.
The clinical follow-up revealed that in the CRT group 19/470 (4.0%) and in the no CRT group 40/1068 (3.6%) developed a LR. In nine patients with LR, no data regarding use of CRT could be obtained. Of the 68 patients, 15 were treated with curative intent, whereas the remaining 53 were referred to palliative treatment. Of the 15 patients curatively treated for local recurrences, 5 developed a later recurrence at any site.
shows the results of regression analysis for the risk of local recurrence. In the crude analysis, both R-status and histopathological stage III show a statistically significant increased risk of local recurrence. In the adjusted analysis, both variables remain statistically significant.
Table 2. Factors influencing local recurrence.
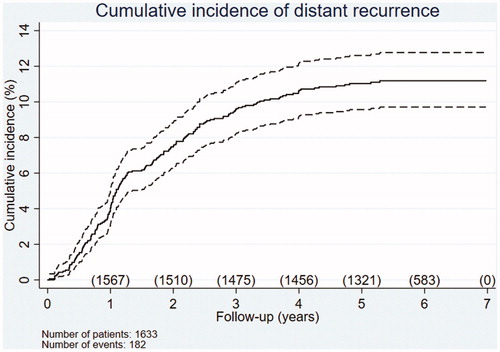
A total of 158 patients developed distant metastases as the first site of recurrence, and 24 patients developed local and distant recurrence synchronously, yielding a total of 182 patients (11.4%). shows the cumulative incidence of distant metastases over time. Of these 182 patients, 94 were referred to palliative care. The remaining 88 patients were treated with curative intent, 53 of whom were continuously followed without later re-recurrence.
Figure 3. Cumulative incidence of distant recurrence during the follow-up period. The number of patients at risk is shown per year. Of the 1633 patients with RC, 158 developed distant recurrence as a first site and 24 developed local and distant recurrence synchronously as a first site, yielding a total of 182 occurrences (11.4%). Dotted lines represent 95% confidence intervals.
Of the remaining 35 patients with re-recurrence (5 local re-recurrences), 10 underwent curative treatment again, whereas the other 25 were referred to palliative treatment.
For distant recurrences, regression analysis was also performed (see Supplementary Table B) using (y)pT and (y)pN variables. In crude analysis, both pN1 and pN2 were statistically significant. In addition, both variables significantly increased the risk of distant recurrences based on the adjusted analysis (adjusted HR 4.32, 95% CI 3.00–6.21 for pN2).
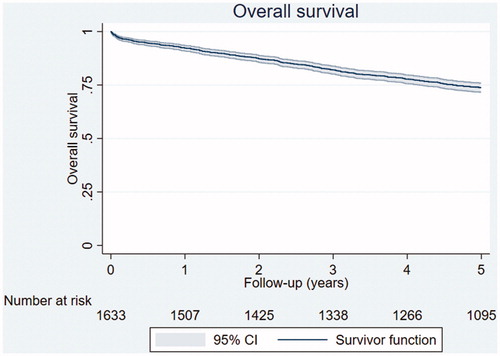
shows the OS for all 1633 RC patients included. The five-year OS was 74% (95% CI: 72–76).
Figure 4. Five-year OS for the 1633 included patients with RC (74%, 95% CI: 72–76). The number of patients at risk is shown per year.
Patients with separate follow-up
Of the 35 patients with synchronous CC, 33 patients had resection of both primary RC and CC, and three of these patients developed local recurrence at the site of RC during follow-up.
Of the 159 patients with metastatic disease at diagnosis, 90 were referred to palliative care. The remaining 69 were treated with curative intent; 35 developed a recurrence, and 6 of them had a local recurrence.
Discussion
The present study is based on baseline characteristics from the DCCG.dk database [Citation23]. In its current form, no clinical follow-up data are registered in this database, which necessitated chart reviews for all included patients. It would be greatly advantageous to additional registry studies if more follow-up data, including data for local and distant recurrences, were registered or collected from other registries.
Compared to other international reports, our study revealed comparable figures for the total LRR in patients curatively treated for RC when Danish CRT guidelines were applied, which consequently resulted in fewer patients treated with CRT. Of interest, our recent Danish figure for a cumulative LRR (4.2%) is close to those reported in both Norway (5%) and Sweden (4%) [Citation22].
The results in are quite similar to those obtained in the TME trial with a 12-year follow-up [Citation6]. Thus, our results are aligned with others, showing that the LRR is mostly evident after at least five years of follow-up.
Approximately one-third of the patients who developed local recurrence had primary high RC. These patients were described as having a fairly good prognosis and were often treated with only surgical procedures and less extensive surgery in terms of partial mesorectal excision [Citation27,Citation28]. This less extensive treatment schedule might reflect the relatively high number of local recurrences, which has also been described by others [Citation29–31]. However, a better approach could be more extensive surgery in terms of TME to remove a sufficient amount of the mesorectum in the upper part of the rectum. Two previous studies have shown contradictory results regarding preoperative radiotherapy for high RC [Citation4,Citation5]. Thus, the role of preoperative CRT remains uncertain for high RC.
Our finding that local recurrence occurred in 19 out of 68 patients despite preoperative CRT was in agreement with previous publications [Citation32]. These tumors may show primary radio resistance [Citation33]. Local recurrences may be avoided with improved tumor and patient characteristics.
The results showed an increased risk of local recurrence for patients receiving a R1-resection. This finding emphasizes the importance of proper diagnostic staging to achieve an R0-resection. Thus, patients with a threatened margin should be considered for preoperative CRT to avoid a local recurrence. The UICC stage displayed in is significantly lower in patients treated with CRT, which may likely be explained by down staging. Patients with stage III show a significantly increased risk for local recurrence, which is in line with other publications and has led many societies to recommend CRT for all patients preoperatively staged as stage III [Citation1,Citation2]; however, MRI-based preoperative staging has shortcomings concerning the identification of involved lymph nodes [Citation34].
The frequency of distant metastasis is lower than that reported in Norway and Sweden, who have a five-year distant recurrence rate of approximately 18% [Citation22]. This discrepancy is difficult to explain, but it might be explained by differences in follow-up schedules and patient attendance. For distant recurrence, pN1 and pN2 significantly raised the risk of recurrence. As described in the literature, postoperative adjuvant chemotherapy is a controversial matter in RC [Citation35]. Further intensified neoadjuvant treatment schedules might be a promising way to decrease this risk [Citation36,Citation37].
In the present study, a relatively high five-year OS was obtained. This result could be explained by the strict selection of patients with curatively intended treatment of RC only, thus excluding patients with metastatic disease and palliative surgery.
In all, when comparing the results from Norway to those from the present study, a more restrictive use of long-course preoperative CRT could be justified. However, the use of preoperative CRT worldwide has been widened considerably, changing from the prevention of local recurrence in resectable tumors and conversion of non-resectable to resectable lesions only to the inclusion of a strategy of organ-sparing with minimal or no surgical procedures [Citation38]. Now, the most interesting question is who among these patients with small lesions could be candidates for preoperative CRT, which reveals the urgent need for predicting response to CRT.
The results obtained in this study confirm the findings of other reports, that with modern RC management, distant recurrences remain a greater issue than local recurrences [Citation14,Citation39]. New treatment schedules have therefore focused on increasing the use of full-dose chemotherapy preoperatively in clinical trials [Citation37].
Strength and limitations: A strength of this study is its consecutive design with a complete national investigation of a large cohort of patients with RC with appropriate follow-up times and a low drop-out rate.
A limitation might be the local recurrences that occurred late after distant recurrence. Patients may have been referred to palliative care, and local tumor control is often assessed only in case of symptoms. This scenario could affect the true LRR, but the applied method attempted to include all clinically important recurrences. In addition, patients with distant recurrences could be missed due to limited follow-up.
In conclusion, a five-year follow-up of curatively treated patients with RC in Denmark revealed a low LRR. The figures are identical to those reported from other Nordic countries, despite considerably stricter Danish guidelines for CRT. In our opinion, the obtained results justify the currently adopted restrictive use of preoperative CRT in Denmark.
Supplemental Material
Download Zip (266 KB)Acknowledgments
The authors wish to thank Martin Berg Johansen for performing the statistical analyses.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv72–iv83.
- Benson AB, Bekaii-Saab T, Chan E, et al. Rectal cancer. J Natl Compr Canc Netw. 2012;10:1528–1564.
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193.
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646.
- Sebag-Montefiore DJ, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet 2009;373:811–820.
- van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–582.
- Braendengen M, Tveit KM, Bruheim K, et al. Late patient-reported toxicity after preoperative radiotherapy or chemoradiotherapy in nonresectable rectal cancer: results from a randomized Phase III study. Int J Radiat Oncol Biol Phys. 2011;81:1017–1024.
- Bruheim K, Tveit KM, Skovlund E, et al. Sexual function in females after radiotherapy for rectal cancer. Acta Oncol. 2010;49:826–832.
- Bruheim K, Guren MG, Dahl AA, et al. Sexual function in males after radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2010;76:1012–1017.
- Folkesson J, Engholm G, Ehrnrooth E, et al. Rectal cancer survival in the Nordic countries and Scotland. Int J Cancer. 2009;125:2406–2412.
- Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery – the clue to pelvic recurrence? Br J Surg. 1982;69:613–616.
- Heald RJ, Moran B, Ryall RD, et al. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978. Arch Surg 1998;133:1997894–1997899.
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740.
- Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with t3 rectal cancer: trans-tasman radiation oncology group trial 01.04. JCO 2012;30:3827–3833.
- DCCG.dk Årsrapport 2013 Landsdaekkende database for kraeft i tyk- og endetarmen [in Danish]. Available from: https://dccg.dk/wp-content/uploads/2017/10/Aarsrapport_2013.pdf
- Van Den Broek CBM, Van Gijn W, Bastiaannet E, et al. Differences in pre-operative treatment for rectal cancer between Norway, Sweden, Denmark, Belgium and the Netherlands. Eur J Surg Oncol. 2014;40:1789–1796.
- Guren MG, Kørner H, Pfeffer F, et al. Nationwide improvement of rectal cancer treatment outcomes in Norway, 1993-2010. Acta Oncol. 2015;54:1714–1722.
- Kodeda K, Johansson R, Zar N, et al. Time trends, improvements and national auditing of rectal cancer management over an 18-year period. Colorectal Dis. 2015;17:O168–O179.
- DCCG.dk Neoadjuverende behandling af resektabel rectumcancer [in Danish]. 2014. Available from: http://dccg.dk/retningslinjer/20140418/2014_NeoAdjRectum.pdf.
- Peeters KCMJ, van de Velde CJH, Leer JWH, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients – a Dutch colorectal cancer group study. JCO 2005;23:6199–6206.
- Birgisson H, Påhlman L, Gunnarsson U, et al. Late adverse effects of radiation therapy for rectal cancer – a systematic overview. Acta Oncol. 2007;46:504–516.
- Glimelius B, Myklebust TÅ, Lundqvist K, et al. Two countries – two treatment strategies for rectal cancer. Radiother Oncol. 2016;121:357–363.
- Ingeholm P, Gögenur I, Iversen LH. Danish colorectal cancer group database. CLEP 2016; 8:465–468.
- DCCG.dk. Surgical Guidelines 2009–2010 [in Danish]. Available from: https://dccg.dk/wp-content/uploads/2017/08/Retningslinier2009p.pdf.
- DCCG.dk Adjuverende kemoterapi ved rectumcancer [in Danish]. 2014. Available from: http://dccg.dk/retningslinjer/indeks.html.
- Aalen O, Johansen S. An empirical transition matrix for non-homogeneous Markov chains based on censored observations. Scand J Stat. 1978;5:141–150.
- Lopez-Kostner F, Lavery IC, Hool GR, et al. Total mesorectal excision is not necessary for cancers of the upper rectum. Surgery 1998;124:612–617.
- Nesbakken A, Nygaard K, Westerheim O, et al. Local recurrence after mesorectal excision for rectal cancer. Eur J Surg Oncol. 2002;28:126–134.
- Syk E, Torkzad MR, Blomqvist L, et al. Radiological findings do not support lateral residual tumour as a major cause of local recurrence of rectal cancer. Br J Surg. 2006;93:113–119.
- Bondeven P, Hagemann-Madsen RH, Laurberg S, et al. Extent and completeness of mesorectal excision evaluated by postoperative magnetic resonance imaging. Br J Surg. 2013;100:1357–1367.
- Bondeven P, Laurberg S, Hagemann-Madsen RH, et al. Suboptimal surgery and omission of neoadjuvant therapy for upper rectal cancer is associated with a high risk of local recurrence. Colorectal Dis. 2015;17:216–224.
- Syk E, Torkzad MR, Blomqvist L, et al. Local recurrence in rectal cancer: anatomic localization and effect on radiation target. Int J Radiat Oncol Biol Phys. 2008;72:658–664.
- Lim SH, Chua W, Henderson C, et al. Predictive and prognostic biomarkers for neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Crit Rev Oncol Hematol. 2015;96:67–80.
- Poulsen LØ, Yilmaz MK, Oddershede L, et al. Is the accuracy of preoperative MRI stage in rectal adenocarcinoma influenced by tumour height? Acta Oncol. 2018;57:728–734.
- Poulsen LØ, Qvortrup C, Pfeiffer P, et al. Review on adjuvant chemotherapy for rectal cancer – why do treatment guidelines differ so much? Acta Oncol. 2015;54:437–446.
- Bujko K, Wyrwicz L, Rutkowski A, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27:834–842.
- Nilsson PJ, van Etten B, Hospers GA, et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer – the RAPIDO trial. BMC Cancer.2013;13:279.
- Appelt AL, Pløen J, Harling H, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16:919–927.
- Rödel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979–989.
