The rare concurrence of pregnancy and lung cancer presents a significant clinical challenge. In younger patients, the presence of molecular driver mutations is more frequently observed [Citation1], but treatment experience with newer targeted therapies during pregnancy is extremely limited. Here, we present a patient who was diagnosed with anaplastic lymphoma kinase (ALK) rearranged non-small cell lung cancer (NSCLC) and received treatment with the ALK inhibitor crizotinib during pregnancy.
Case report
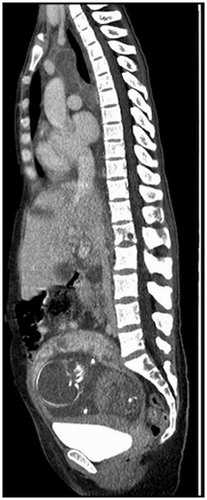
In late August 2016, a 32-year old-pregnant woman at gestational age (GA) 20 + 4 presented with a persistent cough and fever. After radiological and pathoanatomical workup, the patient was diagnosed with metastatic pulmonary adenocarcinoma with ALK rearrangement (). Treatment with crizotinib was initiated in early September. The patient was discussed weekly by a multidisciplinary team comprising oncologists, obstetricians, palliative staff and a pediatrician. After three weeks of treatment, at GA 26 + 0, a CT scan showed stable disease with a 5% size reduction of target lesions. However, at this point the patient developed edema and a biochemical workup showed elevated liver enzymes, thrombocytopenia and albuminuria. Due to suspected HELLP syndrome (hemolysis, elevated liver enzymes and low platelets), a cesarean section was performed. A baby girl was delivered at GA 26 + 2, weight 700g corresponding to −28%, APGAR 5/1, 9/5 and 10/10.
Figure 1. Diagnostic low dose sagittal CT scan demonstrating malignant involvement of the mediastinum, liver and L1 vertebrae as well as the presence of a growing fetus in utero.
Subsequently, symptoms progressed with respiratory insufficiency, septic shock and cerebral infarction. Crizotinib was discontinued. The patient passed away three days after the cesarean section.
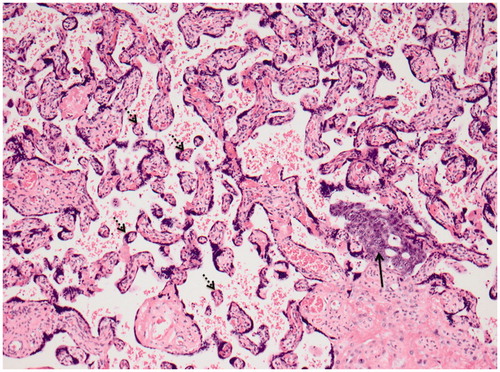
An umbilical cord blood sample was analyzed. The concentration of crizotinib was below the limit of quantification and approximately 4 ng/ml after extrapolation. Pathoanatomical examination of the placenta revealed metastases from the patient’s lung adenocarcinoma as well as signs of maternal vascular malperfusion consistent with clinical HELLP syndrome ().
Figure 2. Placental tissue with distal villous hypoplasia showing small hypermature chorionic villi with increased syncytial knots (stipled arrows) in a widened intervillous space containing a group of tumor cells (black arrow) from the patient’s lung carcinoma (HE ×100).
Due to the risk of transplacental transmission, the child has been subject to frequent follow up visits. The most recent pediatric assessment was conducted 18 months following the cesarean section and demonstrated normal development.
Discussion
Crizotinib is primarily metabolized by the liver and has an approximate biological half-life of 42 hours [Citation2]. In the present case, the concentration of crizotinib in the umbilical cord was extremely low. This could imply low placental transfer of crizotinib, contrasting the findings of an ex vivo study that demonstrated rapid accumulation of crizotinib in placental cotyledons [Citation3]. Furthermore, crizotinib is a substrate of the multidrug resistance p-glycoprotein (P-gp) [Citation4]. P-gp is expressed in the human placenta, which might explain the low concentration observed. No analysis was conducted on maternal blood. Thus, the observed low concentration may reflect decreased maternal absorption or increased metabolism of the drug. Another explanatory factor could be placental hypoperfusion secondary to HELLP syndrome. Only one comparable case report has been published [Citation5]. Here, a 36-year-old woman with NSCLC received crizotinib throughout the entire duration of pregnancy before giving birth to twins. A two-year follow up revealed no abnormalities.
The development of HELLP syndrome is a well-known complication of pre-eclampsia, a common condition of pregnancy defined by the onset of proteinuria and hypertension. Various thrombogenic conditions are associated with an increased risk of developing pre-eclampsia [Citation6]. As malignant disease also carries an increased risk of thrombus formation, the patient’s lung cancer was a probable contributing factor in the development of HELLP syndrome. To our knowledge, treatment with crizotinib has not been compellingly shown to confer an increased risk of thromboembolic events and seems unlikely to comprise a risk factor for the development of pre-eclampsia.
Conclusively, though evidence is sparse, treatment with crizotinib during pregnancy has so far not resulted in abnormal fetal development.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Dagogo-Jack I, Gainor JF, Porter RL, et al. Clinicopathologic features of NSCLC diagnosed during pregnancy or the peripartum period in the era of molecular genotyping. J Thorac Oncol. 2016;11:1522–1528.
- Kwon J, Meagher A. Crizotinib: a breakthrough for targeted therapies in lung cancer. J Adv Pract Oncol. 2012;3:267–272.
- Eliesen GAM, van den Broek P, van den Heuvel JJ, et al. Highlight: placental disposition and effects of crizotinib: an ex vivo study in the isolated dual-side perfused human cotyledon. Toxicol Sci. 2017;157:500–509.
- Tang SC, Nguyen LN, Sparidans RW, et al. Increased oral availability and brain accumulation of the ALK-inhibitor crizotinib by coadministration of the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Int J Cancer. 2014;134:1484–1494.
- Su HI, Connell MW, Bazhenova LA. Ovarian stimulation in young adult cancer survivors on targeted cancer therapies. Fertil Steril. 2016;106:1475–1478.
- Bartsch E, Medcalf K, Park A, et al. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ. 2016;353:i1753.
