Abstract
Background: Survivin is an inhibitor of apoptosis, and its expression associates with poor outcomes in multiple cancers. It may be a therapeutic target due to its unique expression in cancer cells.
Methods: We estimated the association between nuclear and cytoplasmic survivin expression in primary tumors and breast cancer recurrence. In this case-control study, we included women age 35–69, diagnosed with stage I–III breast cancer between 1985 and 2001, and registered with the Danish Breast Cancer Group. We identified 541 patients with breast cancer recurrence with estrogen receptor–positive disease who were treated with tamoxifen for at least 1 year (ER+/TAM+) and 300 with estrogen receptor–negative carcinomas, not treated with tamoxifen, and who survived at least 1 year (ER−/TAM−). Controls were matched to cases on ER/TAM status, date of surgery, menopausal status, stage and county. Survivin expression was estimated by immunohistochemistry on tissue microarrays. We fit logistic regression models to estimate odds ratios (ORs) and 95% confidence intervals (CIs) associating nuclear and cytoplasmic survivin expression with recurrence.
Results: Associations between nuclear and cytoplasmic survivin expression and breast cancer recurrence were near-null in both ER+/TAM + and ER−/TAM − strata. For the cytoplasmic to nuclear ratio (CNR) of survivin expression, we found a null association in the ER+/TAM + group comparing CNR ≥5 with CNR <5, but an association (OR =2.48, 95% CI: 1.15, 5.31) in the ER−/TAM − group.
Conclusions: Survivin expression was not associated with breast cancer recurrence in this study. The CNR ratio may warrant further investigation especially among ER − tumors.
Introduction
With the exception of non-melanoma skin cancer, breast carcinoma is the most commonly diagnosed malignancy among women and it remains the second leading cause of cancer-related death [Citation1]. Guideline-based therapy for breast cancer has been tailored to the expression of tumor biomarkers, such as estrogen receptor alpha (ERα), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), which has improved survival and reduced breast cancer recurrence risk [Citation2,Citation3]. While stratified therapy has improved outcomes, 20%–30% of women diagnosed with breast cancer develop recurrent disease within 10 years of initial diagnosis [Citation4]. The search continues for tumor biomarkers that are predictive of endocrine therapy resistance and indicative of patient prognosis to further improve breast cancer outcomes and reveal opportunities for developing new therapeutic agents [Citation3].
Dysregulation of proteins involved in cell division, proliferation, differentiation and apoptosis, characterize tumorigenesis [Citation5,Citation6]. In cell cycle regulation, a cascade of enzymes either promotes or counteracts apoptosis. One important group in the regulation of apoptosis is the IAP (inhibitor of apoptosis) protein family, which includes survivin [Citation6,Citation7]. The genes encoding these proteins have been identified across species and are highly evolutionarily conserved [Citation8,Citation9]. Survivin inhibits caspase enzymes thereby blocking apoptosis, although the exact mechanism is not well understood [Citation10]. Whilst survivin is widely expressed during fetal development, it is down-regulated during adult life, becoming (with few exceptions) undetectable in most normal terminally differentiated tissues [Citation10,Citation11]. In contrast, survivin expression has been observed in most types of human malignancy, suggesting that the protein plays a key role in cancer progression [Citation12]. This strikingly specific association with cancer has attracted attention to survivin as a possible treatment target, and has led to the development of a number of agents designed to inhibit survivin expression [Citation13].
In tumor cells, survivin appears in two different nuclear-cytoplasmic pools and it has been hypothesized that the different localizations might reflect different functions of the protein [Citation7,Citation14]. Survivin expression has primarily been reported in the cytoplasm from studies using immunohistochemistry and has been associated with poor prognosis [Citation15]. However, some studies have reported nuclear expression of survivin, which is hypothesized to be a marker of a more favorable prognosis [Citation16]. Fifteen previous publications have examined survivin expression by immunohistochemistry among women diagnosed with breast cancer in relation to disease-free survival and overall survival [Citation17,Citation18]. However, these studies may be limited by sample sizes of less than 200 participants and lack of control for potential confounders.
Tamoxifen is an adjuvant endocrine therapy prescribed to women diagnosed with ER+ breast carcinomas, reducing the risk of recurrence by nearly 50%. Efforts to identify biomarkers that are predictive of resistance to tamoxifen therapy are crucial for improving breast cancer outcomes. Survivin may provide insight into tamoxifen resistance and breast cancer prognosis based on its role in proliferation and inhibition of apoptosis. In the current investigation, we evaluated the value of nuclear and cytoplasmic survivin expression as a predictive marker of resistance to endocrine therapy with tamoxifen, and a prognostic marker of breast cancer recurrence. We performed a large, population-based case-control study with control for demographic, treatment and tumor characteristics.
Material and methods
Study population
This study used data from the Danish Breast Cancer Group (DBCG), a population-based clinical registry that has collected data on nearly all breast cancer patients in Denmark since 1977 [Citation19,Citation20]. The source population consisted of 11,251 female residents of the Jutland Peninsula in Demark with a primary breast cancer diagnosis of stage I, II, or III as defined by the Union for International Cancer Control (UICC) [Citation21]. Women were included if their diagnosis occurred between 35 and 69 years of age, and if they were registered in the DBCG clinical registry.
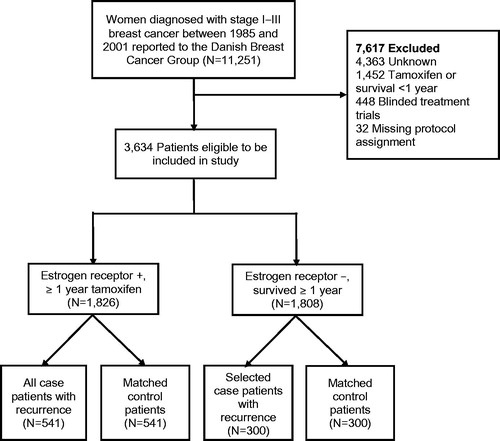
The source population was divided into two groups: women whose tumor expressed ER and who were treated with tamoxifen for at least one year (ER+/TAM+, n = 1826), and those whose tumor did not express ER, were not treated with tamoxifen, and who survived for at least one year (ER−/TAM−, n = 1808). Women who did not fit either designation (i.e., whose tumors expressed ER but were not treated with tamoxifen, or whose tumors did not express ER but were treated with tamoxifen) were excluded (n = 7617). We stratified the analyses by ER/TAM status to separate a potential prognostic effect of survivin expression from a potential predictive effect of survivin expression modifying tamoxifen effectiveness. Follow-up time was calculated from one year after breast cancer diagnosis until the first of [Citation1] breast cancer recurrence, [Citation2] death from any cause, [Citation3] loss to follow-up, [Citation4] completion of 10-year follow-up, or [Citation5] 1 September 2006.
Cases were defined as women with a diagnosis of local or distant breast cancer recurrence or contralateral breast cancer occurrence during follow-up. Among the ER+/TAM + group, 541 women met the case definition, and all were included. Among the ER−/TAM − group, we selected 300 cases, whose frequency matched according to the distribution of stage and calendar period of diagnosis among the ER+/TAM + patients. For each case, one control was selected without replacement from members of the source population, who were not diagnosed with a breast cancer recurrence or contralateral breast cancer occurrence by the time of the matched case’s recurrence. Controls were matched to cases based on group (ER+/TAM + or ER−/TAM−), menopausal status at diagnosis, date of breast cancer surgery (caliper matching +/–12 months), county of residence at time of diagnosis, and cancer stage at diagnosis ().
Data collection from Danish registries
Every Danish resident is assigned a unique 10-digit Civil Personal Registration (CPR) number, which allows accurate individual-level linkage of patient information across databases [Citation22]. Using the CPR numbers of the patients selected for our study, we collected data from the DBCG registry, including information on demographics (age, menopausal status and hospital of diagnosis), tumor (UICC stage, histological grade and ER expression), and treatment characteristics (primary surgical tumor management, receipt of radiation therapy, receipt of chemotherapy and receipt of tamoxifen therapy).
Data collection from archived tissue samples
Laboratory personnel were blinded to all clinical information including case or control status, ER status and receipt of tamoxifen therapy.
Tissue microarray construction and immunohistochemistry
For each case and control, formalin-fixed, paraffin-embedded primary tumor tissue blocks were retrieved from the pathology archives of treating hospitals. In Denmark, all paraffin blocks from pathological specimens are kept permanently after being used for diagnosis. Patient CPR-number were used to link to the Danish Pathology Data Bank [Citation23], enabling us to locate and retrieve suitable tumor blocks from nearly all study subjects (1512 of 1682, 90%).
Tissue microarrays (TMAs) were constructed using standard techniques [Citation24]. A fresh section was cut from each paraffin block in the study, stained routinely with hematoxylin and eosin, and the diagnosis confirmed by a study pathologist. Appropriate tissue regions were identified and marked-up, following which core samples were removed from each tumor donor block and re-embedded in a new recipient paraffin TMA block. If there was sufficient material, representative 1 mm diameter tumor (n = 3) and marginal tissue (n = 1) cores were sampled using a TMA Master arrayer (3DHISTECH Budapest, Hungary). Liver and placental cores were included in each TMA to serve as staining controls, and to facilitate orientation within the TMA during microscopy.
Immunohistochemical stains were performed on 3 µm TMA tissue sections according to standard protocols, optimized in house for use with the Ventana Benchmark ULTRA automated staining system (Ventana Medical Systems, Tucson, AZ). Expression of survivin was assessed using a rabbit monoclonal antibody (Abcam, Cambridge, UK; catalog no. EP2880Y). This antibody was selected after failing to optimize with a mouse monoclonal antibody (Santa Cruz, Ca. #sc-17779) that appeared to have unspecific staining. After review from other laboratories and publications we selected the rabbit monoclonal antibody, which stained specifically and performed well. Antigen retrieval was achieved using Cell Conditioning 1 (CC1, pH 8.5, Ventana) at 97 °C. Sections were then incubated with the primary antibody at a concentration of 2522 µg/µl, which was diluted 1:100 for 32 minutes. Positive reactions were detected with OptiView 3,3′-diaminobenzidine (DAB) IHC detection kit (Ventana) before counterstaining with hematoxylin. Specific staining was characterized by positivity of the nuclei and/or cytoplasm.
Stained TMA sections were scanned at 40× magnification with the Hamamatsu Nanozoomer 2.0HT (Hamamatsu Phototonics KK, Hamamatsu City, Japan) in ndpi format. Resulting files were uploaded to Visiopharm Integrator System software (VIS; Visiopharm A/S, Hoersholm, Denmark) and dearrayed to facilitate manual scoring of individual cores.
TMA core scoring
Survivin expression in invasive breast carcinoma cells was scored on digital images of the individual TMA cores, . Positive immunohistochemical staining was scored separately in nuclei and in the cytoplasm, using a semi-quantitative H-score [Citation25,Citation26] based on both staining intensity and the percentage of positively stained tumor cells. The H-score weights intensity based on a four-level factor: 0 (no staining), 1 (light staining), 2 (medium staining) and 3 (heavy staining). Percent positivity is a continuous metric from 0% to 100% positivity based on the proportion of positively stained tumor cells. Non-tumor cell regions of the core were not included in the eligible area for scoring. These two values are then multiplied for each core to estimate an H-score that ranges from 0 to 300, for both cytoplasmic and nuclear staining. The final H-score for each patient’s tumor was the average of the H-scores from available tumor cores stained and eligible for scoring.
Figure 2. Immunohistochemical analysis of nuclear and cytoplasmic survivin expression. (A) IHC ×10 high cytoplasmic staining intensity, magnified to (B) IHC ×40, 60% high intensity, 40% low intensity. (C) IHC ×10 low/medium nuclear staining intensity, magnified to (D) IHC ×40, 5% medium intensity, 3% low intensity.
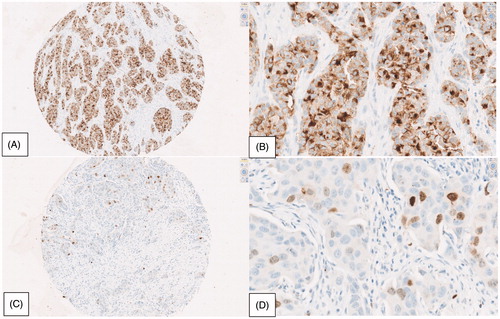
The scoring system used (and agreement on the interpretation of the different intensity levels) was developed in pilot studies involving several observers (data not shown). Subsequently, all study cores were evaluated by one rater (LJC). Cases that were difficult to interpret were reviewed by two observers (LJC and SHD) and a consensus score was agreed. In all, paraffin embedded primary tumor samples were available for TMA construction from 1512 patients (90%). Construction of TMAs from these 1512 blocks generated 4351 cores, of which 3111 (71%) were eligible for scoring. Cores that were not scored were excluded, either because the core section on the TMA was absent or inadequately represented, or because of technically poor staining of the core section.
Analytic variables
Expression of survivin
The exposure of interest for this study was expression of nuclear and cytoplasmic survivin. We operationalized nuclear and cytoplasmic expression as follows. In the first analysis, both nuclear and cytoplasmic expression were dichotomized as ≥50th percentile of the H-score or otherwise. We also analyzed nuclear and cytoplasmic expression in three categories, comparing 50th–75th percentile H-score and >75th percentile H-score with a reference category of <50th percentile of the H-score. Nearly half of the tumor cores showed no survivin expression in the nucleus or cytoplasm, which influenced the choice of category boundaries for this analysis. In a second analysis, we considered the joint expression of nuclear and cytoplasmic staining as a dichotomous variable, comparing presence of both nuclear and cytoplasmic expression with no expression in either of these cellular compartments. Finally, we considered the relationship between nuclear and cytoplasmic staining as the ratio of cytoplasmic H-score to the nuclear H-score, restricted to tumors in which both nuclear and cytoplasmic staining had non-zero values.
Breast cancer recurrence
The outcome was breast cancer recurrence, which was ascertained from the DBCG database. According to the DBCG guidelines, recurrence is defined as any breast cancer or distant metastasis diagnosed after an initial course of therapy [Citation27]. For the purpose of this study, we limited cases to those occurring within 1–10 years of follow-up.
Covariates
We included UICC stage (I, II, III), grade (I, II, III), menopausal status at diagnosis (premenopausal/postmenopausal), receipt of chemotherapy (yes/no), receipt of radiotherapy (yes/no), surgery type (mastectomy/breast conserving surgery), year of diagnosis, age at diagnosis and county of residence in each analysis.
Statistical analysis
Analyses were stratified by the ER/TAM grouping to evaluate whether survivin expression was predictive of tamoxifen resistance, prognostic of breast cancer recurrence, or neither. For descriptive analyses, we calculated the frequency and proportion of cases and controls within categories of nuclear and cytoplasmic survivin expression and in categories of covariates. Unconditional logistic regression was used to estimate the association between survivin expression and breast cancer recurrence, controlling for UICC stage, grade, menopausal status, receipt of chemotherapy, receipt of radiotherapy, surgery type (mastectomy versus breast conserving surgery), diagnosis year, age category at diagnosis and county of residence. All analyses were performed in SAS 9.4 (Cary, NC).
The study was approved by the Danish Data Protection Agency (record number 2015-57-0002 and Aarhus University journal number 2016-051-000001, running number 458), the Danish Ethical Committee (record number 1-10-72-16-15), and the Emory University Institutional Review Board.
Results
In this population-based case control study, we observed 43% of cases and 40% of controls with positive nuclear survivin expression and 52% of cases and 52% of controls with positive staining for cytoplasmic survivin, among those in the ER+/TAM + group (). Nuclear and cytoplasmic survivin expression was observed in 32% of cases and 29% of controls.
Table 1. Distribution of tumor and patient characteristics among breast cancer recurrence cases and controls by ER/TAM group among 1682 subjects in the population-based case-control study of women age 35–69, diagnosed with Stage I – III breast cancer between 1985 and 2001, and registered with the Danish Breast Cancer Group.
Nuclear survivin expression
In the unconditional analyses (), in the ER+/TAM + group, we observed an OR for nuclear survivin expression of 1.14 (95% CI: 0.80, 1.64) comparing 50th–75th percentile expression to <50th percentile, and an OR of 0.91 (95% CI: 0.62, 1.33) comparing >75th percentile to <50th percentile. In the dichotomous classification of nuclear survivin expression, we observed a similar null association comparing ≥50th percentile to <50th percentile. In the ER−/TAM − group, the OR comparing 50th–75th percentile nuclear survivin expression to <50th percentile was 0.89 (95% CI: 0.52, 1.53), and the OR comparing >75th percentile nuclear survivin expression to <50th percentile was 1.27 (95% CI: 0.77, 2.09). The OR was similar in the dichotomous classification of nuclear survivin expression.
Table 2. Association between nuclear and cytoplasmic survivin and breast cancer recurrence from a population-based case-control study of 1312 women age 35–69, diagnosed with Stage I – III breast cancer between 1985 and 2001, and registered with the Danish Breast Cancer Group.
Cytoplasmic survivin expression
In the ER+/TAM + group, we observed an OR for cytoplasmic survivin expression of 1.11 (95% CI: 0.78, 1.56) comparing 50th–75th percentile to <50th percentile and an OR of 0.94 (95% CI: 0.66, 1.34) comparing >75th percentile to <50th percentile (). In the dichotomous classification of cytoplasmic survivin expression, we also observed a null association with recurrence. In the ER−/TAM − group, the OR comparing 50th–75th percentile to <50th percentile was 1.04 (95% CI: 0.64, 1.67) and the OR comparing >75th percentile to <50th percentile was 1.13 (95% CI: 0.69, 1.85).
Nuclear and cytoplasmic survivin expression
In the ER+/TAM + group, we observed an OR of 1.13 (95% CI: 0.83, 1.54) comparing patients with joint expression of nuclear and cytoplasmic survivin to those without. We observed a similar effect estimate in the ER−/TAM − group. With regard to the cytoplasmic to nuclear ratio (CNR) of survivin expression, in the ER+/TAM + group those with a CNR ≥5 had an OR of 1.11 (95% CI: 0.70, 1.77) compared to those with a CNR <5. In the ER−/TAM − group, the OR was 2.48 (95% CI: 1.15, 5.31) comparing those with a CNR ≥5 to those with CNR <5.
Dose response
Additionally, we evaluated the associations between cytoplasmic, nuclear and CNR expression in a dose-response pattern (). In the multivariable logistic regression analyses, these comparisons were near null and without evidence of any important trend. However, the CNR dose response among the ER−/TAM − group did show increasing breast cancer recurrence with increasing CNR, consistent with the dichotomous multivariable analyses.
Table 3. Dose-response evaluation of the association between nuclear and cytoplasmic survivin and breast cancer recurrence from a population-based case-control study of 1312 women age 35–69, diagnosed with Stage I – III breast cancer between 1985 and 2001, and registered with the Danish Breast Cancer Group.
Discussion
Our findings do not support an association between nuclear and cytoplasmic survivin expression in breast carcinoma tissue and breast cancer recurrence in either ER+/TAM + or ER−/TAM− groups, irrespective of whether nuclear and cytoplasmic staining were assessed separately or in combination. We observed an association between the cytoplasmic to nuclear ratio and breast cancer recurrence in the ER−/TAM− group, for which there was greater than two-fold increased odds of recurrence comparing those patients with CNR ≥5 to those with CNR <5. This may indicate that overexpression of cytoplasmic survivin drives the proliferation of ER− negative tumor cells, leading to recurrence. A previous study that estimated the association between CNR and overall and disease-free survival found an inverse association, hazard ratio 0.09 (95% CI: 0.01–0.76), between increasing CNR and overall survival [Citation28]; however, this study had a sample size of only 102 breast carcinomas.
To the best of our knowledge, our study is the largest to date, to study the predictive and prognostic role of survivin expression in breast tumor tissue, and one of ∼10 studies to study the potential association with breast cancer recurrence. In two recent meta-analyses that pooled 3259 and 2202 breast cancer patients respectively, the summary hazard ratios (HRs) for the role of survivin expression were estimated as HR =1.80 (95% CI: 1.55, 2.09) and HR 1.37 (95% CI: 1.12, 1.68) for overall survival and HR =1.34 (95% CI: 1.02, 1.76) for disease-free or relapse-free survival, suggesting that increased expression of survivin is associated with worse breast cancer outcomes [Citation17,Citation29]. Among studies that assessed survivin expression with IHC, the pooled effect estimate for disease-free survival was 1.42 (95% CI: 0.72, 2.82), which aligns with our study results [Citation29]. The studies included in these analyses had a median sample size of 142 breast cancer patients and median 33 outcomes, compared with 541 ER+ recurrences and 300 ER− recurrences in our study. The use of IHC to assess survivin expression provides an assessment of the amount of translated protein and has important clinical potential. mRNA analysis may, however, have higher sensitivity, but not all of the mRNA will be translated to protein expression, which is more accurately captured by IHC. These earlier studies also incompletely adjusted for tumor and patient characteristics that are considered potentially important confounders of the association between survivin expression and breast cancer survival, whereas we were able to adjust for many prognostic characteristics.
Our study has some limitations. Half of the breast tumor tissues showed no expression of survivin in the nucleus or cytoplasm. While this pattern is consistent with other studies and accords with the biology of survivin expression [Citation7,Citation30], it led to some small cell counts in the categorization of survivin expression. Since survivin is generally not observed in normal adult human tissues, except transiently during mitosis, but is expressed in most types of cancer, there has been considerable interest in the protein as a prognostic biomarker, and a possible therapeutic target. We did not confirm this potential in this analysis, although the observed association between the CNR ratio and breast cancer recurrence among ER− tumors may be of importance. Additionally, study participants consisted mainly of women diagnosed with Stage II (48%) and Stage III (48%) breast cancers, which is largely a result of the DBCG criteria for tamoxifen therapy during the period of diagnosis of the study population [Citation31].
In addition to being a large population-based study, established using validated and comprehensive health-care registries, our study has several strengths. Breast cancer recurrence data were validated in a previous validation study using medical record review, thereby reducing the potential for important misclassification of the outcome [Citation32]. Review of medical records did identify some discrepancies with respect to length of tamoxifen treatment, with most patients actually receiving longer durations of tamoxifen therapy compared with the indicated duration registered in the DBCG [Citation32]. This pattern is most likely the result of providers switching to longer protocols during the study period as evidence grew favoring a 5-year protocol. However, this would not affect our results.
In conclusion, in this population-based nested case-control study, we did not confirm previous findings suggesting that survivin is a prognostic marker for breast cancer recurrence. Ours is the first population-based study to examine possible predictive and prognostic associations between survivin expression and the risk of breast cancer recurrence. The role of survivin may be related to mortality rather than recurrence, although it is difficult to understand how survivin expression would affect mortality without affecting recurrence in early stage breast cancer patients. Further investigation into the ratio of cytoplasmic to nuclear survivin expression, especially among women with ER− tumors, in relation to breast cancer recurrence may be warranted.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66:7–30.
- Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies—improving the management of early breast cancer: St Gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015;26:1533–1546.
- Gradishar WJ, Anderson BO, Balassanian R, et al. Invasive breast cancer version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14:324–354.
- Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–1691.
- Cross M, Dexter TM. Growth factors in development, transformation, and tumorigenesis. Cell. 1991;64:271–280.
- Deveraux QL, Reed JC. IAP family proteins-suppressors of apoptosis. Genes Dev. 1999;13:239–252.
- Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921.
- Shin S, Sung B-J, Cho Y-S, et al. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry. 2001;40:1117–1123.
- Dubrez-Daloz L, Dupoux A, Cartier J. IAPs: more than just inhibitors of apoptosis proteins. Cell Cycle. 2008;7:1036–1046.
- Mita AC, Mita MM, Nawrocki ST, et al. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5005.
- Zhao J, Tenev T, Martins LM, et al. The ubiquitin-proteasome pathway regulates survivin degradation in a cell cycle-dependent manner. J Cell Sci. 2000;113:4363–4371.
- Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54.
- Ryan BM, O'Donovan N, Duffy MJ. Survivin: a new target for anti-cancer therapy. Cancer Treat Rev. 2009;35:553–562.
- Fortugno P, Wall NR, Giodini A, et al. Survivin exists in immunochemically distinct subcellular pools and is involved in spindle microtubule function. J Cell Sci. 2002;115:575–585.
- Athanassiadou AM, Patsouris E, Tsipis A, et al. The significance of survivin and nectin-4 expression in the prognosis of breast carcinoma. Folia Histochem Cytobiol. 2011;49:26–33. Epub 2011/04/29.
- Kennedy S, O'Driscoll L, Purcell R, et al. Prognostic importance of survivin in breast cancer. Br J Cancer. 2003;88:1077–1083.
- Song J, Su H, Zhou YY, et al. Prognostic value of survivin expression in breast cancer patients: a meta-analysis. Tumor Biol. 2013;34:2053–2062. Epub 2013/05/22.
- O'Driscoll L, Linehan R, SMK, Cronin D Purcell R, Glynn S, et al. Lack of prognostic significance of survivin, survivin-deltaEx3, survivin-2B, galectin-3, bag-1, bax-alpha and MRP-1 mRNAs in breast cancer. Cancer Lett. 2003;201:225–236. Epub 2003/11/11.
- Jensen M-B, Laenkholm A-V, Offersen BV, et al. The clinical database and implementation of treatment guidelines by the Danish Breast Cancer Cooperative Group in 2007–2016. Acta Oncol. 2018;57:13–18.
- Ejlertsen B, Offersen BV, Overgaard J, et al. Forty years of landmark trials undertaken by the Danish Breast Cancer Cooperative Group (DBCG) nationwide or in international collaboration. Acta Oncol. 2018;57:3–12.
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. Chichester, West Sussex, UK; Hoboken, NJ: John Wiley & Sons; 2011.
- Schmidt M, Pedersen L, Sørensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549.
- Erichsen R, Lash TL, Hamilton-Dutoit SJ, et al. Existing data sources for clinical epidemiology: the Danish national pathology registry and data bank. Clinical Epidemiol. 2010;2:51–56. Epub 2010/09/25. PubMed PMID: 20865103; PubMed Central PMCID: PMCPMC2943174.
- Kononen J, Bubendorf L, Kallionimeni A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847.
- Allred DC, Harvey JM, Berardo M, et al. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. Epub 1998/03/21.
- McCarty KS, Jr., Miller LS, Cox EB, et al. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109:716–721. Epub 1985/08/01.
- Andersen KW, Mouridsen H. Danish breast cancer cooperative group (DBCG): a description of the register of the nation-wide programme for primary breast cancer. Acta Oncol. 1988;27:627–647.
- Brennan DJ, Rexhepaj E, O'Brien SL, et al. Altered cytoplasmic-to-nuclear ratio of survivin is a prognostic indicator in breast cancer. Clin Cancer Res. 2008;14:2681–2689.
- Li Y, Ma X, Wu X, et al. Prognostic significance of survivin in breast cancer: meta-analysis. Breast J. 2014;20:514–524. Epub 2014/07/22.
- Tanaka K, Iwamoto S, Gon G, et al. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res. 2000;6:127–134.
- Møller S, Jensen M-B, Ejlertsen B, et al. The clinical database and the treatment guidelines of the Danish breast cancer cooperative group (DBCG); its 30-years experience and future promise. Acta Oncol. 2008;47:506–524.
- Lash TL, Cronin-Fenton D, Ahern TP, et al. CYP2D6 inhibition and breast cancer recurrence in a population-based study in Denmark. J Natl Cancer Inst. 2011;103:489–500.