Introduction
Adjuvant radiotherapy (RT) following breast-conserving surgery (BCS) or postmastectomy RT has shown improved local control, disease-free, and overall survival of breast cancer patients [Citation1–4]. Improved survival with RT among breast cancer survivors, however, poses risks for late radiation-related cardiac and pulmonary toxicities because of the anatomical proximity of the heart and lung to radiation targets, such as whole breast or chest wall. Patients with unfavorable cardiac–thoracic anatomy (CTA) [Citation5] or chest wall deformity such as pectus excavatum, or those requiring internal mammary node (IMN) RT are at risk of increased radiation dose to the heart and lung. This increased radiation dose to the heart and lung may cause non-breast cancer related mortality and reduce the survival benefits from breast cancer RT [Citation2,Citation6,Citation7].
With technological improvements in the radiation oncology field, advanced RT techniques such as deep inspiration breath hold (DIBH), intensity-modulated RT (IMRT), volumetric arc therapy (VMAT), prone position RT, and proton RT have been applied for certain patients when conventional three-dimensional conformal RT (3DCRT: supine tangential RT in particular) alone could not achieve an efficient and safe breast cancer RT plan. Although advanced RT techniques have shown decreased radiation dose to the heart and lung compared to 3DCRT [Citation8,Citation9], unlike supine tangential RT, there is limited accessibility for both patients and radiation oncology clinics to these advanced RT techniques. Consequently, not all patients who require breast cancer RT can get benefits from these recent technological advances.
Continuous positive airway pressure (CPAP) is a ventilator providing low-to-moderately positive air pressure on a continuous basis to keep the airways open. CPAP has been widely used for patients with obstructive sleep apnea. Compared to free-breathing (FB), CPAP can make the thorax continuously more inflated which can subsequently displace the heart away from chest wall and decrease breathing-caused thoracic movements by limiting breathing span. These physical changes in the thorax with CPAP can potentially lower the radiation dose to the heart and lung as well as reduce radiation target (whole breast or chest wall) movement during breast cancer RT.
In this study, we aimed to evaluate the physical changes in the thorax with CPAP in comparison to FB, and dosimetrically compare breast cancer RT plans using conventional supine tangential fields for patients with favorable, unfavorable, and challenging CTA (F-, UF-, and CH-CTA, respectively).
Material and methods
Patients
Three patients were selected for this study, a patient with invasive ductal carcinoma in right breast who underwent BCS (pT1N0M0) and two patients with left breast cancers who underwent mastectomy (pT2N1M0 of invasive ductal carcinoma and pT2N1M0 of invasive lobular carcinoma). All three patients were not able to maintain and reproduce deep enough breath hold for application of the DIBH technique for their breast cancer RT: one patient with left breast cancer and another patient with right breast cancer have had a long history of cigarette smoking and developed shortness of breath after only a few DIBH exercises; the other patient with left breast cancer had pectus excavatum, which limited her deep inspiration (). Based on the authors’ experiences using CPAP during breast cancer RT [Citation10], all three patients were recommended to receive RT with CPAP.
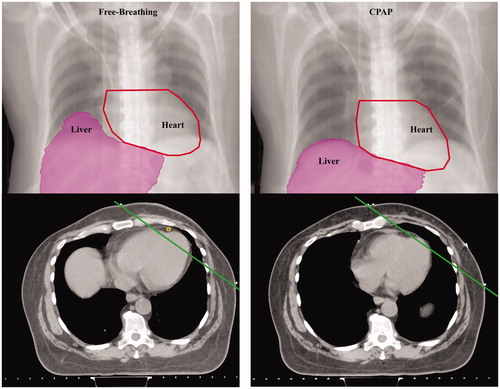
Figure 1. Physical changes in the thorax with continuous positive airway pressure (CPAP) compared to free-breathing in patients with different cardiac–thoracic anatomy. Digitally reconstructed radiographs (top) and axial images (bottom) from computed tomography simulation scans representative thoracic changes with free-breathing and after 30 min of CPAP breathing under the individual maximum tolerable air pressure (between 6 and 15 cmH2O with 0.21 of fraction of inspired oxygen with the use of a mask interface) in patients with different cardiac–thoracic anatomy (CTA). Unfavorable CTA was defined per Taylor et al.,5 the maximum distance >1 cm between the heart contour and the posterior edge of the tangent field as measured on computed tomography simulation with FB. Chest wall deformity with pectus excavatum was considered as challenging CTA. If the patient did not meet the two descriptions stated above, the patient was categorized as favorable CTA. Panel A, B, and C shows patient with favorable-, unfavorable-, and challenging CTA, respectively (Supplementary Figures S1 A and B with favorable- and challenging CTA, respectively).
Computed tomography-simulation
Before computed tomography (CT)-simulation, all patients visited the CPAP clinic to find the individual maximum tolerable air pressure between 6 and 15 cmH2O with 0.21 of fraction of inspired oxygen with the use of a mask interface. All patients underwent CT simulation with FB (CT-FB) in the supine position on a Vac-Lok bag on a breast board followed by 30 min of CPAP breathing under the maximum tolerable air pressure. A repeat CT simulation with CPAP (CT-CPAP) was done on the same day with the patient in the same position as the CT-FB. Radiation therapists set up the CPAP for the patients according to the CPAP clinic guidance during all procedures. UF-CTA was defined per Taylor et al. [Citation5], the maximum distance >1 cm between the heart contour and the posterior edge of the tangential fields as measured on CT-FB. Chest wall deformity with pectus excavatum was considered as CH-CTA. If the patient did not meet the two descriptions stated above, the patient was categorized as F-CTA.
Radiotherapy plan
Planning target volumes (PTVs) and organs at risk (OARs) were contoured on both CT-CPAP and CT-FB scans for RT planning (ipsilateral-RT plan) according to RTOG 1304. Contralateral side whole breast or chest wall were also contoured on both CT-CPAP and CT-FB to create contralateral-RT plans for dosimetric comparison for all patients. Prescription was 50 Gy in 25 fractions without boost for all RT plans in this study. Eclipse RT planning system (version 10.0, Varian Medical System, Palo Alto, CA, USA) was used with a Varian Trilogy linear accelerator using 6-MV photons. Treatment planning aimed to reduce dose to OARs as much as possible without compromising the coverage of the PTVs. All RT plans were created using 3DCRT technique for this study. Ipsilateral-postmastectomy RT plans comprised the 4-field photon only fields consisted of two tangential fields covering PTV-chest wall and IMN, and anterior-posterior fields covering PTV-supraclavicular node. For ipsilateral-breast RT and all contralateral-RT plans, two tangential fields covering PTV-whole breast or chest wall only were used. The beam’s-eye view was used to shape multi-leaf collimators to block OARs. Field-in-field technique was used to maximize dose homogeneity.
Dosimetric comparison
Plan quality was determined by PTV and PTV for evaluation (PTV_eval for whole breast or chest wall) structure coverage and dose to the OARs according to RTOG 1304. Percentage change (% change) between FB and CPAP, in dosimetric parameters, was calculated using the formula: % change = (parameter with FB – parameter with CPAP)/parameter with FB.
Statistics
Statistical analysis of dosimetric comparison between RT plans was done using a Student’s t-test. Data are presented as mean ± standard deviation. A probability level of a p value of <.05 was considered significant.
Results
Patients were able to tolerate up-to 10–12 cmH2O of CPAP air pressure. Physical changes in the thorax with CPAP are shown in and . Compared to FB, CPAP with the individual maximum tolerable air pressure significantly inflated the thorax and increased total lung volume (4064 ± 579 cm3 with CPAP versus 3041 ± 496 cm3 with FB; p = .01): 2199 ± 328 versus 1597 ± 297 cm3 in the right lung (p = .04); 1865 ± 256 versus 1444 ± 218 cm3 in the left lung (p = .002).
Table 1. Physical changes in the thorax with continuous positive airway pressure compared to free-breathing in patients with different cardiac–thoracic anatomy.
CPAP displaced the heart more inferiorly than with FB, which subsequently increased distance from the sternal notch to the superior portion of heart by 0.5–1.25 cm with CPAP in comparison to FB. With an inflated thorax and more inferiorly displaced heart with CPAP, the heart volume within the left-sided tangential fields was substantially decreased by more than 92% in comparison to FB regardless of patient’s CTA or IMN RT. Notably, compared to FB, CPAP almost completely excluded the heart from tangential fields in F-CTA (, with UF-CTA, and Supplementary Figures S1(A,B) with F- and CH-CTA, respectively).
Inflated thorax with CPAP also displaced the liver, at least 2 cm, more inferiorly in comparison to FB. The inferiorly displaced liver with CPAP decreased the liver volume within the right-sided tangential fields in comparison to FB in all patients, with the most marked change in F-CTA (84% reduction).
Coverage for radiation targets by RT plans with FB and CPAP are displayed in a Supplementary Table S1. All RT plans demonstrated acceptable PTV or PTV_eval structure coverage per RTOG 1304.
Dosimetric comparison of RT plans with CPAP and FB is shown in . In both right- and left-sided RT plans, CPAP increased lung volume and decreased radiation dose to the ipsilateral lung in all patients regardless CTA or IMN RT. Most substantial changes were noticed in CH-CTA requiring IMN RT: 25% reduction in mean dose (Dmean) and 26% reduction in volume of lung receiving at least 20 Gy (V20).
Table 2. Continuous positive airway pressure effects on radiation doses to organs at risks compared to free-breathing in patients with different cardiac–thoracic anatomy.
Inferiorly displaced heart with CPAP compared to FB also decreased radiation dose to the heart in all left-sided RT plans. The reduction of Dmean to the heart with CPAP was substantially greater for patients with IMN RT (2.9 versus 11.3 Gy in UF-CTA; 0.9 versus 4.8 Gy, in CH-CTA) than the patient without IMN RT (2 Gy versus 2.6 Gy in F-CTA). Compared to FB, CPAP also decreased V25 to the heart by more than 84% in all patients regardless of CTA or IMN RT.
Physical changes in the liver with CPAP decreased the maximum dose to the liver in right-sided RT plans by more than 50% compared to FB in F- and UF-CTA.
Discussion
In this study, we evaluated the effects of CPAP with conventional supine tangential RT on breast cancer RT plans in patients with F-, UF-, and CH-CTA. We demonstrated that CPAP inflated the thorax, displaced the heart and liver away from RT fields, and increased lung volume in the thorax resembling physical changes in the thorax from DIBH in all patients, which substantially and efficiently decreased radiation dose to the heart, lung, and liver compared to FB with conventional supine tangential RT regardless of patients’ CTA or IMN RT.
A recent systematic review reported radiation exposure of the heart in left-sided breast cancer RT using various modern RT techniques [Citation8]. Average Dmean to the heart was higher in patients with unfavorable thoracic anatomy than favorable thoracic anatomy (7.1 versus 4.2 Gy), and with IMN RT (8.4 Gy) than without IMN RT (4.2 Gy). For patients that received left-sided RT including IMN, Dmean to the heart also varied depending on the RT techniques: 9.4 Gy in tangential RT without breathing control; 4 Gy in tangential RT with breathing control; 7.6 Gy in wide tangential RT; 8.5 Gy in IMRT (standard); 8.8 Gy in IMRT (rotational); and 2.6 Gy in proton RT. In this study, we demonstrated that CPAP with left-sided wide tangential RT including IMN can lower Dmean to the heart to 2.9 and 0.9 Gy in patients with UF- and CH-CTA, respectively. These doses are comparable or lower to what could be achieved in a proton RT plan.
Aznar et al. [Citation9] also reported a systematic review of radiation dose to the lung for breast cancer RT using various RT techniques. Similar to the heart, including IMN in the RT field nearly doubled Dmean to ipsilateral lung (7.9 versus 15 Gy). Average Dmean to ipsilateral lung without breathing control were 15 Gy in wide tangential RT, 12.4 Gy in IMRT (supine), 9.4 Gy in IMRT (prone), and 8.7 Gy in proton RT. Breathing control during breast cancer RT including IMN decreased Dmean to ipsilateral lung by 3 Gy. Interestingly, there was no differences in V20 to ipsilateral lung between IMRT and tangential RT not including IMN. However, when IMN was in the RT field, IMRT led to a substantially lower V20 to ipsilateral lung than wide tangential RT. In this study, authors showed that CPAP with supine tangential RT can markedly reduce both Dmean and V20 to ipsilateral lung in all patients regardless of CTA or IMN RT. Notably, with CPAP, we were able to meet acceptable Dmean and V20 to ipsilateral lung dose constraints in left-sided wide tangential RT plans including IMN in UF-CTA (V20: 36%) and CH-CTA (Dmean: 18 Gy; V20: 36%), otherwise those dosimetric parameters would be unacceptable with FB.
Although advanced RT techniques in breast cancer have shown dosimetric benefits of decreased radiation dose to the heart and lung over supine tangential RT alone [Citation8–10], these advanced RT techniques are often associated with intense staff- and resource-requirements, and increased capital expenditure in radiation oncology clinics. The availability of these advanced RT techniques commonly depends on the infrastructural constraints in each radiation oncology clinic where breast cancer RT would be performed. Patients with physical restrictions might not be eligible for some of the advanced RT techniques. Breathing control including DIBH requires patients’ compliance, special equipment to detect patients’ breathing, and longer treatment time than RT with FB alone. For certain patients due to their CTA, prone position showed no effect or increased radiation dose to the heart in comparison to the supine position [Citation11,Citation12]. Inverse planning IMRT or VMAT can improve PTV coverage and dose homogeneity (specifically for patients with UF- or CH-CTA, or who require IMN RT) with the cost of increased low-dose radiation to the OARs (heat, lung, and contralateral breast). High-precision RT (IMRT, VMAT, and proton RT) poses risk of intrinsic uncertainty of radiation dose to moving radiation target, which may cause over- or under-dosing PTV edge and interfacing OARs, particularly the heart and lung in breast cancer RT. Those intrinsic uncertainty can be greater when PTV moves with large breathing span which would require patients’ breathing control during RT. Financial burden due to using advanced RT techniques can also limit patients’ accessibility to the advanced RT techniques. Medicare estimated costs for breast cancer RT using IMRT or proton therapy were more than twice that of 3DCRT [Citation13–15]. Because the treatment-related cost must be economically sustainable, cost becomes an important factor to take into consideration on decision making for cancer treatment modality.
As described in this report, physical changes in the thorax with CPAP made it possible to achieve the heart and ipsilateral lung dose constraints using only supine wide tangential fields instead of complex photon–electron match or advanced RT techniques in UF- and CH-CTA requiring IMN RT. Additionally, a more inferiorly displaced liver with CPAP in comparison to FB can reduce unnecessary radiation exposure to the liver in right-sided RT plans. Limiting patients’ breathing span with CPAP compared to FB can also minimize breathing-caused thoracic movements, thus resulting in radiation target (whole breast or chest wall) becoming more stable and suitable not only for 3DCRT, but also for high-precision RT (IMRT, VMAT, or proton RT) when 3DCRT fails to achieve an effective and safe RT plan. With its easy set-up, CPAP has been already widely used for the patients with sleep apnea at home. Thus, CPAP can be easily implemented in daily radiation oncology practice as it was done in our clinic to provide the heart and lung sparing breast cancer RT with less infrastructural and financial burden on patients, radiation oncology clinics, and the health care system.
Conclusion
The heart and lung sparing breast cancer RT is possible with conventional supine tangential fields using CPAP regardless of patients’ CTA or IMN RT. Findings in this report suggest that CPAP with conventional supine tangential RT can be an alternative method to reduce radiation dose to OARs for patients or radiation oncology clinics with limited accessibility to the advanced RT techniques.
Supplemental Material
Download Zip (1.1 MB)Acknowledgments
This material is the result of work supported with resources and the use of facility at the Oklahoma City Veterans Affairs Health Care System, Oklahoma City, Oklahoma.
Disclosure statement
No potential conflict of interest was reported by the authors. The contents do not represent the views of the US Department of Veterans Affairs or the United States Government. The Oklahoma City Veterans Affairs Health Care System’s institutional review board waived a board review on the case report. The patients have given signed consent for reporting this report.
References
- Thorsen LB, Offersen BV, Danø H, et al. A population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer. J Clin Oncol. 2016;34:314–320.
- McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomized trials. Lancet. 2014;21:2127–2135.
- Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:1816–1877.
- Early Breast Cancer Trialists’ Collaborative Group. Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. N Engl J Med. 1995;333:1444–1455.
- Taylor C, McGale P, Povall JM, et al. Estimated cardiac exposure from breast cancer radiotherapy in clinical practice. Int J Radiat Oncol Biol Phys.2009;73:1061–1068.
- Darby SC, Ewertz M, Hall P. Ischemic heart disease after breast cancer radiotherapy. N Engl J Med. 2013;368:2527.
- Taylor C, Correa C, Duane F, et al. Estimated risks of breast cancer radiotherapy: evidence from modern radiation doses to the lung and heart and from previous randomized trials. J Clin Oncol. 2017;35:1641–1649.
- Taylor C, Wang Z, Macaulay E, et al. Exposure of the heart in breast cancer radiation therapy: a systematic review of heart doses published during 2003 to 2013. Int J Radiat Oncol Biol Phys. 2015;93:845–853.
- Aznar M, Duane F, Darby SC, et al. Exposure of the lungs in breast cancer radiotherapy: a systematic review of lung doses published 2010–2015. Radiother Oncol. 2018;126:148–154.
- Kil WJ, Pham T, Hossain S, et al. The impact of continuous positive airway pressure on radiation dose to heart and lung during left-sided postmastectomy radiotherapy when deep inspiration breath hold technique is not applicable: case report. Radiat Oncol J. 2018;36:79–84.
- Formenti S, DeWyngaert K, Jozsef G, et al. Prone vs supine positioning for breast cancer radiotherapy. JAMA. 2012;308:861–863.
- Kirby AM, Evans PM, Donovan EM, et al. Prone versus supine positioning for whole and partial-breast radiotherapy: a comparisons of non-target tissue dosimetry. Radiother Oncol. 2010;96:178–184.
- Smith BD, Pan IW, Shih YC, et al. Adoption of intensity-modulated radiation therapy for breast cancer in the United States. J Natl Cancer Inst. 2011;103:798–809.
- Shah C, Lanni TB, Saini H, et al. Cost-efficacy of acceleration partial-breast irradiation compared with whole-breast irradiation. Breast Cancer Res Treat. 2013;138:127–135.
- Ovalle V, Strom EA, Godby J, et al. Proton beam partial breast irradiation for early stage breast cancer: a not-so-costly alternative. Paper presented at the Particle Therapy Co-Operative Group of North America 1st Annual Meeting; 2014 October 27–29; Houston, TX.
