Abstract
Introduction: There is a paucity of data on incidence and mechanisms of long-term gastrointestinal consequences after chemoradiotherapy for anal cancer. Most of the adverse effects reported were based on traditional external beam radiotherapy whilst only short-term follow-ups have been available for intensity-modulated radiotherapy, and there is lack of knowledge about consequences of dose-escalation radiotherapy.
Method: A systematic literature review.
Results: Two thousand nine hundred and eighty-five titles (excluding duplicates) were identified through the search; 130 articles were included in this review. The overall incidence of late gastrointestinal toxicity was reported to be 7–64.5%, with Grade 3 and above (classified as severe) up to 33.3%. The most commonly reported late toxicities were fecal incontinence (up to 44%), diarrhea (up to 26.7%), and ulceration (up to 22.6%). Diarrhea, fecal incontinence and buttock pain were associated with lower scores in radiotherapy specific quality of life scales (QLQ-CR29, QLQ-C30, and QLQ-CR38) compared to healthy controls. Intensity-modulated radiation therapy appears to reduce late toxicity.
Conclusion: Late gastrointestinal toxicities are common with severe toxicity seen in one-third of the patients. These symptoms significantly impact on patients’ quality of life. Prospective studies with control groups are needed to elucidate long-term toxicity.
Introduction
The incidence of anal cancer has been increasing for the past 30 years. In English National Cancer Data Repository, 13,940 patients were identified between 1990 and 2010 with a primary diagnosis of anal cancer, with a significant increase in squamous cell anal carcinoma [Citation1]. And more than 8500 new cases are expected in 2018 in USA [Citation2]. The first-line treatment of squamous cell carcinomas of anal canal has evolved from surgery to curative chemoradiotherapy, due to superior local control, survival and preservation of the anal sphincter function as demonstrated in phase III trials [Citation3–5]. Chemoradiotherapy with 5-fluoruracil (5-FU) and mitomycin C (MMC) is considered to be the gold standard in the treatment of anal cancer. [Citation6]. Currently, surgery is reserved for salvage [Citation3,Citation7].
Pelvic radiotherapy has some disadvantages. It may lead to a spectrum of acute and late toxicities, such as bleeding, diarrhea, or skin problems. There has been extensive reporting on acute toxicities, particularly in the setting of rigorous clinical trials. However, late complications of radiotherapy that occur beyond the setting of trials have been poorly recorded. Some of the adverse effects that occur after the completion of trials, such as fecal incontinence, urgency, anal stenosis, and evacuation difficulty of anal cancer survivors, have not received much attention to date [Citation8].
The aim of this review was to collate the current knowledge of late gastrointestinal toxicity associated with chemoradiotherapy for anal cancer by conducting a systematic literature review. The objectives were to define the prevalence and severity of gastrointestinal symptoms, and the physiology and pathology of anal canal after irradiation to elucidate the possible mechanisms of the adverse symptoms, and to evaluate various therapeutic options that have been used.
Method
Search strategy
PubMed database was searched, using the primary keywords ‘anal cancer’, ‘radiotherapy’, ‘fecal incontinence’, ‘urgency’, ‘rectal bleeding’, and ‘evacuation difficulty’ for English-language articles published from January 1980 to March 2018. A search using each keyword was performed, followed by a combination of two of them and finally all three keywords. In the second step, each combination of two primary keywords was combined with one of the following secondary keywords: ‘pathology’, ‘complication’, ‘rectal morbidity’, ‘anal morbidity’, ‘late reaction’, ‘acute reaction’, ‘toxicity’, ‘fecal urgency’, and ‘radiation’ to allow broad and comprehensive yet sufficiently focused search of the literature.
Titles and abstracts were screened permissively, such that a study was included if it had any indication of being eligible. The articles selected for full text review were evaluated as to whether they satisfied the inclusion and exclusion criteria by two authors (Y.P., Y.M.). Data were extracted to an Excel file to collate figures for incidence and frequency of adverse events.
Included studies were those regarding primary radiotherapy for anal cancer and reporting mechanisms and tissue damage descriptions of fecal incontinence, rectal bleeding, evacuation difficulties (including anal stenosis/stricture), radiation proctopathy/proctitis (enteritis, mucositis), fistula communicating with gastrointestinal tract.
Studies were excluded if they were reporting radiotherapy for a primary cancer other than anal cancer, surgical operative techniques or complications of surgical operations for anal cancer, those focusing on the technical aspects of radiotherapy with no report on adverse effects, secondary malignancy as a consequence of radiotherapy, non-gastrointestinal complications relating to radiotherapy for anal cancer (e.g. urinary incontinence, urinary retention, renal failure and sexual dysfunction, myelosuppression, skin erythema and/or desquamation), palliative radiotherapy and certain types of articles such as case reports, letters and comments, review and seminar articles, and literature written in languages other than English.
The review was undertaken in accordance with the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [Citation9].
Definition of acute and late GI toxicity
According to literature, the most frequently used toxicity assessment tools were the national cancer institute Common Terminology Criteria for Adverse Events (CTCAE) classification [Citation10] and the Radiation Therapy Oncology Group (RTOG/EORTC) radiation morbidity scoring scheme [Citation11].
There were 13 symptoms defined as lower GI adverse events in CTCAE v3.0, namely colitis, constipation, diarrhea, fistula, hemorrhage, incontinence anal, mucositis, necrosis, obstruction, proctitis, perforation, stricture/stenosis, and ulcer. In the updated version (CTCAE v4.0), more GI adverse symptoms were added such as diarrhea, fistula, hemorrhage, necrosis, obstruction, perforation, stricture/stenosis, and ulcer. The site of these symptoms were also added with ‘anal’ or ‘rectal’ in front.
Toxicity in CTCAE is graded as mild (Grade 1), moderate (Grade 2), severe (Grade 3), or life-threatening (Grade 4), with specific parameters according to the organ system involved. Death (Grade 5) is used in some of the criteria to denote a fatality. The timing of adverse event is not defined in both versions of the CTCAE.
In the identified articles, the definition of timing was variable. Most of the reported timing of adverse events (AE) was between 2 and 6 months, and they used 3 months as the cutoff to separate acute and late toxicity [Citation12].
The Radiation Therapy Oncology Group (RTOG/EORTC) radiation late morbidity scoring scheme grades toxicity that occurred 90 days after the commencement of treatment [Citation11]. The RTOG/EORTC morbidity criteria grade severity by symptoms into four grades. For example, rectal bleeding of Grade 1 is ‘slight’, Grade 2 is ‘intermittent’, and Grade 3 is ‘bleeding requiring surgery’. Grade 4 includes necrosis, perforation and fistula. Grades 3 and above toxicities have often been grouped as ‘major’ toxicities [Citation13].
Results
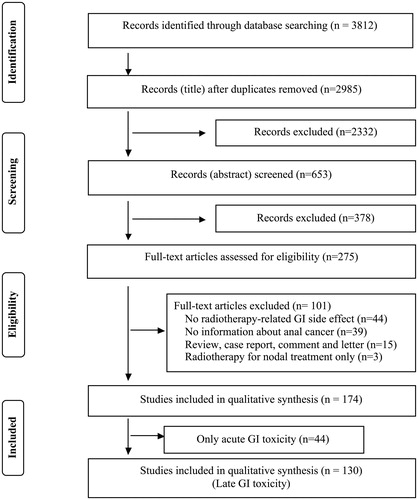
Two thousand nine hundred and eighty-five titles (excluding duplicates) were identified through the search of which 174 articles fulfilled the criteria. Forty-four articles out of 174 papers referred to acute GI toxicity (symptoms during or up to three months following chemoradiotherapy) only. As we focused on the late gastrointestinal (GI) toxicity in this review, these papers were excluded. One hundred and thirty papers were included for this review. The study selection process is illustrated in .
Incidence of late GI toxicity
Overview
The overall incidence of any late GI adverse events after radiotherapy for anal cancer was reported to be 7–64.5% [Citation14–19].
Out of 130 papers, 116 papers reported all adverse events (regardless of grade), 14 papers reported only adverse events Grade 3 or above. The incidence of Grade 1 and 2 toxicities were 26.4–50%, whilst that of Grade 3 and above were reported to occur between 0 and 33.3% of patients [Citation4,Citation20–26]. Eighteen papers reported only the incidence of overall GI adverse events with no data for individual GI symptom [Citation4,Citation27–30].
Fecal incontinence
The reported rate of FI following radiotherapy ranged, from 0 to 44% [Citation18,Citation31–42]. Between 0.5 and 10.9% of anal cancer survivors were reported to have needed a colostomy due to severe anal sphincter dysfunction at a median follow-up of 32.4 months to 8.6 years [Citation43–51].
There were only five cohort or retrospective studies which specifically assessed the consequence or complications after chemoradiotherapy for anal cancer. These studies used more specific fecal incontinence assessment tools such as anorectal manometry, quality of life measurement and St. Mark’s incontinence score [Citation52]. The rate of FI was higher in these five papers, ranging from 38 to 44% [Citation31,Citation53–56].
Assessment
One of the standard assessments of fecal incontinence is anorectal manometry, which measures sphincter pressures of internal and external anal sphincters. Only three papers used anorectal manometry to assess the function of the anal sphincter. The anal cancer survivors were compared with young, healthy volunteers in two papers [Citation31,Citation54], and with age-and sex-matched control subjects hospitalized for minor operations unrelated to anal cancer in one paper [Citation53]. The numbers of included patients were very small in these three papers (n = 8, 10, and 16, respectively). There was no study that performed anorectal manometry before and after chemoradiotherapy.
The resting pressure and the maximum squeeze pressure were lower in patients who received chemoradiotherapy, compared to those in the control group [Citation31,Citation53]. The details are summarized in . Radiation may also affect the sensitivity to rectal distension. One study showed the sensation threshold volume to rectal distension was 36% lower whilst maximum tolerable volume was 45% higher in irradiated patients compared to that of controls (p = .05) [Citation53]. However, other studies did not show any differences in volumes compared to control groups [Citation31,Citation54].
Table 1. Results of anorectal manometry after radiotherapy for anal cancer.
Radiotherapy technique
The incidence of FI in patients who were treated with intensity-modulated radiotherapy (IMRT) was 0–4.6% [Citation57,Citation58], whilst those patients who were treated with three dimensional external beam radiotherapy (3D-EBRT) had an incidence of FI ranging from 3.7 to 12% [Citation59,Citation60]. There has been only one retrospective cohort study which compared the outcome of two different radiotherapy techniques: the incidence of severe (Grade 2 or 3 of CTCAE v4.0) FI was 5.4% in 3D-EBRT cohort (97.5 months follow-up), while no Grade 2 or 3 FI occurred in the IMRT cohort (30.8 months follow-up) [Citation61].
Treatment
One study reported on the use of topical phenylephrine, a selective alpha1-agonist which causes internal sphincter contraction and raises resting pressure: overall the median St. Mark’s incontinence score improved from 17 [interquartile range (IQR) 14–20] before the treatment to 14 (IQR 11–18) after the treatment (p = .005). However, there was only one anal cancer patient included in this series and the outcome of this specific patient was not available [Citation62].
Another paper used sacral nerve stimulation for FI in four anal cancer patients out of 13 pelvic radiotherapy patients. In total, seven patients (54%) had successful percutaneous nerve evaluation (PNE), with the number of incontinence episodes in the 3-week bowel diary reduced from median of 24 (range 4–56) to 4 (range3–6) [Citation63].
There have been no reports about other currently available treatments for FI (e.g. anal plug, constipating agents, biofeedback, antegrade/retrograde irrigation) specifically for post-radiotherapy FI.
Ulceration
The reported incidence of ulceration as a late complication was between 1.2–22.6%, and 2.3–7.6% of chronic ulcer needed a surgical intervention [Citation3,Citation64–70]. Two early small cases studies reported that ulcers led to formation of anal stricture (1 out of 30 and 1 out of 12, respectively) [Citation71,Citation72].
A retrospective analysis showed patients with HIV-positive status had the higher risk of developing skin toxicities [post-treatment ulcer: 43.8% (HIV-positive) versus 18.1% (HIV-negative), p = .02]. HIV status was also the only factor that predicted late severe skin and digestive toxicities (35.3 versus 14.8%, p = .04) [Citation73]. But another paper support the rate of long-term side effects was low in HIV-seropositive patients [Citation74].
Diarrhea
The incidence of diarrhea varied from 0 to 26.7% [Citation75–81]. The definition of diarrhea varied such as ‘severe diarrhea required therapy’ and ‘drug-resistant chronic diarrhea’ and some studies reported only Grade 3 and above chronic diarrhea [Citation64,Citation82]. The incidence of severe or ≥ Grade 3 diarrhea (increase of ≥7 stools per day over baseline in CTCAE, stool frequency >8/day in LENT-SOMA), range from 0.4 to 4.9% [Citation64,Citation83–85].
There has been mixed views with regards to the relationship between HIV infection status and the incidence of diarrhea [Citation75,Citation86,Citation87]. A few studies reported diarrhea scores of anal cancer patients were worse compared to volunteers (p < .001) [Citation8,Citation55,Citation88]. However, the severity of diarrhea using scores has not been reported to be different before and after the treatment [Citation89,Citation90].
An RCT study concluded that the prophylactic use of long-acting octreotide did not prevent the incidence or reduce the severity of diarrhea and had no notable impact on patient-reported bowel function or quality of life [Citation91].
Radiation proctopathy/proctitis/mucositis
The incidence of late radiation proctopathy or proctitis ranged from 0 to 40% [Citation92–97]. In CTCAE criteria, the definition of proctitis is unclear and likely to be overlapped with other late toxicities, because Grade 1 is ‘rectal discomfort’, Grade 2 is ‘passing blood or mucus’, and Grade 3 is ‘stool incontinence’. Radiation proctopathy or proctitis or mucositis is not mentioned in RTOG/EORCT and LENT-SOMA criteria.
The incidence does not appear to be different between radiotherapy modalities [Citation64,Citation98]. Two studies reported the use of formalin in treating chronic radiation-induced hemorrhagic proctitis. One study (15 patients, including two anal cancer patients) showed that 87% patients had complete cessation of bleeding [Citation99]. One prospective study (33 patients including 11 anal cancer) also stated that formalin was effective treatment for chronic radiation-induced hemorrhagic proctitis, but not suitable for anal cancer survivors due to the increased morbidities of anal stricture and FI [Citation100].
Other GI toxicities
Other symptoms of late toxicities include chronic hemorrhage or bleeding. One of the earlier papers (58 patients) published in 1993 stated that ‘Almost all patients are expected to have occasional rectal bleeding after a firm bowel movement’ [Citation101]. With the evolution of radiotherapy techniques, the incidence has become lower: Grade 1 and 2 bleeding up to 34.5% and Grade 3 to 4 bleeding late toxicity between 0.4 and 25.4% [Citation83,Citation84]. A randomized, double-blind, sham-controlled phase 3 trial studied the clinical benefits of hyperbaric oxygen in patients with chronic bowel dysfunction after radiotherapy for pelvic malignancies (84 cases, including eight anal cancer). It showed no significant differences in the change of IBDQ rectal bleeding score [median change from baseline to 12 months of 3 (1–3) in the treatment group versus 1 (1–2) in the sham group; U-score 1·69, p=·092] [Citation102].
Anal stenosis or stricture occurs in up to 8.5% of patients and 0.4–4.4% of them will require dilation or operative intervention [Citation83,Citation103,Citation104]. Anal or perineal fibrosis is reported in up to 50% [Citation72] and it can affect not only the targeted anal canal (1.5–26.7%) [Citation101,Citation103,Citation105] but also rectum (0.5%) [Citation76], intestine (2.2%) [Citation77], vagina (2.9–8%) [Citation57,Citation106], urethra (6.7%) [Citation75], and skin fibrosis [Citation107], and may contribute to a form of dysfunction of these organs. Fistulae including recto-vaginal, rectovesical, anal, or rectal fistula occur in 0.7–22.2% of patients [Citation75,Citation105,Citation108–112]. Other reported adverse symptoms included perineal/anal/rectal pain, necrosis, colonic obstruction, small bowel obstruction, perforation, telangiectasia, constipation, abdominal pain, tenesmus, enteritis, rectal mucus, and perianal numbness [Citation36,Citation66,Citation84,Citation107,Citation113–118].
It is not uncommon that multiple late toxicities occur at the same time. One toxicity may also lead to another toxicity, such as fibrosis that causes stricture or formation of an ulcer and subsequent healing can also lead to a stricture.
In the final analysis of the randomized UNICANCER ACCORD 03 Trial, 9 out of 307 patients (2.9%) needed abdomino-perineal resection or diversion colostomy for severe gastrointestinal toxicities such as necrosis, fistulae, bleeding, or pain [Citation84].
Long-term QOL of anal cancer survivors after radiotherapy
Assessment of quality of life after radiotherapy was performed using were QLQ-C30 [Citation119,Citation120], QLQ-CR38 [Citation121,Citation122], QLQ-CR29 [Citation89,Citation123], and GIQLI [Citation124].
Patients’ QoL scores were often significantly worse at the end of treatment but recovers by 3 months, except for fecal incontinence and diarrhea [Citation89,Citation107]. Cross-sectional studies reported that patients treated for anal cancer had significantly worse long-term QOL (e.g. inferior global QOL, buttock pain, fecal incontinence, and sexual problems) compared to healthy volunteers [Citation8,Citation11,Citation103,Citation125,Citation126].
Patients who survived anal cancer have significantly more gastrointestinal symptoms such as fecal incontinence, anal/perianal pain, rectal bleeding or stool frequency which are persistent up to 5 years [Citation8,Citation54,Citation56,Citation88,Citation125]. Some affected the overall quality of life score [Citation121].
It may be difficult to untangle whether the change in the quality of life is due to the results of late toxicities. Whilst a few studies found strong correlation with QLQ-CR38 and late gastrointestinal toxicity symptoms [Citation64,Citation126], others found only moderate correlation between fecal incontinence score and global QOL [Citation55].
Radiotherapy technique and dosage and their influence on late toxicity
It is known that using 3D conformal radiotherapy and more recently intensive modulation radio therapy (IMRT) have reported significant improvement in acute toxicity without compromising cancer treatment effect [Citation127,Citation128].
Studies reporting the long-term outcome of IMRT reported either no or very rare incidence of Grade 3 or higher late sequelae [Citation14,Citation58,Citation61,Citation97,Citation98,Citation129–131]. Three studies found only the gross tumor volume (GTV) dose to be predictive of acute Grade 2 GI toxicity [Citation132–134]. A retrospective study showed a trend toward fewer late complications among the patients treated with a dose of <60 Gy: the late toxicity rate was 14% for a dose <60 Gy compared to 37% for a dose of ≥60 Gy (p = .04) [Citation36]. Another retrospective study also showed late gastrointestinal toxicity was significantly higher in those patients who received a RT dose of >60 Gy compared to those who received <60 Gy (p < .001) [Citation135].
RTOG 9208 assessed an escalation of the radiotherapy dose to 59.6 Gy over 8.5 weeks with a mandatory 2 week gap. This schedule did not appear to improve local control, but showed an increased 1–2 year colostomy rate due to higher local recurrence (1-years 23%, 2-year 30%) compared to the RTOG 87-04 outcomes (1-year 6%, 2-years 7%) where patients received a dose of only 45–50.4 Gy in 1.8 Gy per fraction [Citation5].
Discussion
Late effects develop months or years after chemoradiotherapy. The symptoms may be mild or severe, self-limiting, or progressive, and may develop gradually or suddenly. Late effects tend to occur in tissues with a low turnover of cells, such as subcutaneous tissue, fatty tissue, muscle, and at sites of slow turnover within tissues that contain rapidly proliferating cells, such as the wall of the intestine [Citation136]. Therefore, it is not surprising to see that a plethora of gastrointestinal toxicities have been reported in literature. However, the data was limited because the majority of the literature comprises retrospective studies not specifically designed to look at late toxicities.
The methods of delivering radiotherapy in anal cancer have evolved over years: EBRT in most of the trials was crudely delivered using two- or three-dimensional approaches, which irradiated a large amount of normal tissues (bladder, bowel, perineal region, inguinal skin) with consistently high doses whilst more recent IMRT is more focused approach confining the therapy to the targeted areas of tumor and elective volumes, while sparing organs at risk. It may also allow dose-escalation to be performed safely. This partly explains the high rates of Grade 3–4 acute gastrointestinal (GI) side effects reported in the Radiation Therapy Oncology Group (RTOG) 98-11 trial (35% Grade 3–4, mainly diarrhea). During the RTOG 0529 trial, there was a transition in practice patterns as the contouring guidelines were published, Radiation Oncologists then ensured that there were less hot spots on plans and more formal sparing of some structures such as external genitalia contours. Although IMRT appears to be less morbid, trials have much shorter follow-up and have used more modest doses of radiotherapy. The number of published articles is small and this is yet to be fully assessed using more accurate assessment tools and longer follow-up.
Long-term fecal incontinence in prostate cancer correlates with the mean dose to the anal-sphincter region, with a cut of doses of >40 Gy conferring more risk [Citation137]. Some studies suggest the dose to the anal sphincter mechanism is more crucial rather than the surface of the anorectum [Citation138,Citation139]. A few studies looked at the lowest 2 cm of the rectum as a model for the anal canal and suggested that poor sphincter control correlates best with doses of at least 53 Gy received by the lateral extent of the anal sphincter region [Citation31,Citation140]. However, most of the trials used 45–50.4 Gy and it is difficult to ascertain the suggested distinctive threshold dose of radiation (e.g. 56 Gy) that might lead to fecal incontinence, poor function, and the eventual need for a de-functioning colostomy [Citation53]. Larger and more advanced cancers are more difficult to control than smaller ones and require higher doses of radiation, which in turn causes more morbidity. Identification the optimal radiotherapy dose, either RT alone or with chemoradiation, has been a challenge. Critically, major differences exist in the treatment schedules (planning volumes and doses), not only between but also within the individual RCTs, partly because of a reliance of early response, either histopathologically or clinically to decide the appropriate total radiation dose.
Despite GI consequences being common after chemoradiotherapy, there is infrequent use of symptom-specific assessment and treatments. For example, incontinence specific scores like St. Mark’s score and Wexner’s score that are widely used in clinical practice were not used in the selected studies. The two studies using St. Mark’s score showed a higher incidence of FI in anal cancer survivors after radiotherapy treatment than other nonspecific assessment tools such as CTCAE and LENT-SOMA. Furthermore, there are a few limitations in LENT-SOMA. For example, a huge severity increase from Grade 3 (persistent use of incontinence pads) to Grade 4 (surgical intervention/permanent colostomy) sphincter control management is found in LENT-SOMA, as is the case in CTCAE criteria. Nowadays, conservative treatment such as biofeedback and minimally invasive surgical interventions such as sacral nerve stimulation in treating FI have been established. It is likely that most patients with a variable degree of incontinence will all fall into Grade 4 and it is not possible to differentiate true Grade 4 who have end-stage fecal incontinence and need a colostomy. One of the landmark studies that established 5-fluororacil/mitomycin and radiotherapy regimen for anal cancer provided limited long-term outcomes which were an overall 3-year colostomy-free survival of 74%, and the very few (14 of 844 patients) colostomies done for late treatment effects [Citation6]. It should be also noted that the mean St. Mark’s score were correlated to the role function and social function compared to global QOL in QLQ-C30 and clearly showed that FI has impact on social interaction and affected by patients’ individual perspectives. A newly published prospective cohort study reported that a majority [124 (68.9%)] of hospitalized patients with serious illnesses (such as malignant neoplasms, class III or IV congestive heart failure etc.) considered bowel and bladder incontinence as health states the same or worse than death [Citation141]. An attempt to develop a specific QOL measure for anal cancer was published recently [Citation142]. Further studies are needed to assess the scale of GI consequences using symptom specific assessment tool and evaluating patients with longer follow-ups and including impact on quality of life.
Anorectal manometry showed the resting pressure and the maximum squeeze pressure were all significantly lower in patients who received chemoradiotherapy, compared to those in the control group. However, there appears to be discordance between the manometric data and the FI symptom reported by patients after anal irradiation as lower pressures did not necessarily correlate to symptomatic fecal incontinence. One of the possible explanations is that the decreased sphincter pressures at manometry remains subclinical as continence is a finely tuned mechanism consisting of mucosal sensitivities, reflex, and sphincter pressures and it is not solely reliant on sphincter strength. Currently, the pathophysiology of FI after radiation is poorly understood. Fibrosis is the most studied structural alteration. However, given that there is a reduction in rectal volumes in irradiated patients, fibrosis in the anal canal may not be the only and direct mechanism but it may affect function such as stool sampling and reflex.
Recommendations for future studies will include the use of a multidimensional assessment protocol, using well-validated measures and standardized pretreatment, post-treatment, and follow-up measurements. More research is needed to gain a full understanding of the complexity and variety in the effects of radiotherapy on the functions of anal canal.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Wilkinson J, Morris E, Downing A, et al. The rising incidence of anal cancer in England 1990-2010: a population-based study. Colorectal Dis. 2014;16:O234–O239.
- Siegel R, Miller K, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
- Northover J, Glynne-Jones R, Sebag-Montefiore D, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br J Cancer. 2010;102:1123–1128.
- Ajani JA, Winter KA, Gunderson LL, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA 2008;299:1914.
- John M, Pajak T, Flam M, et al. Dose escalation in chemoradiation for anal cancer: preliminary results of RTOG 92-08. Cancer J Sci Am. 1996;2:205–211.
- James RD, Glynne-Jones R, Meadows HM, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol. 2013;14:516–524.
- Shridhar R, Shibata D, Chan E, et al. Anal cancer: current standards in care and recent changes in practice. CA Cancer J Clin. 2015;65:139–162.
- Bentzen AG, Balteskard L, Wanderås EH, et al. Impaired health-related quality of life after chemoradiotherapy for anal cancer: late effects in a national cohort of 128 survivors. Acta Oncol. 2013;52:736–744.
- Moher D, Liberati A, Tetzlaff J, et al. Reprint-preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89:873–880.
- National Cancer Institute [Internet]. Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Available from: https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (last accessed 22 December 2013).
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol. 1995;31:1341–1346.
- Cummings BJ, Keane TJ, O’Sullivan B, et al. Epidermoid anal cancer: treatment by radiation alone or by radiation and 5-fluorouracil with and without mitomycin C. Int J Radiat Oncol Biol Phys. 1991;21:1115–1125.
- John M, Flam M, Palma N. Ten-year results of chemoradiation for anal cancer: focus on late morbidity. Int J Radiat Oncol Biol Phys. 1996;34:65–69.
- Janssen S, Glanzmann C, Bauerfeind P, et al. Clinical experience of SIB-IMRT in anal cancer and selective literature review. Radiat Oncol. 2014;9:199.
- Lestrade L, De Bari B, Montbarbon X, et al. Radiochemotherapy and brachytherapy could be the standard treatment for anal canal cancer in elderly patients? A retrospective single-centre analysis. Med Oncol. 2013;30:402.
- Ferrigno R, Nakamura R, Dos Santos Novaes P, et al. Radiochemotherapy in the conservative treatment of anal canal carcinoma: retrospective analysis of results and radiation dose effectiveness. Int J Radiat Oncol Biol Phys. 2005;61:1136–1142.
- Doggett S, Green J, Cantril S. Efficacy of radiation therapy alone for limited squamous cell carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 1988;15:1069–1072.
- Mai SK, Welzel G, Hermann B, et al. Long-term outcome after combined radiochemotherapy for anal cancer – retrospective analysis of efficacy, prognostic factors, and toxicity. Onkologie. 2008;31:251–257.
- De Bari B, Etitia Lestrade L, Pommier P, et al. Could concomitant radio-chemotherapy improve the outcomes of early-stage node negative anal canal cancer patients? A retrospective analysis of 122 patients. Cancer Invest. 2015;33:114–120.
- Janssen S, Meier zu Eissen J, Kolbert G, et al. Anal cancer treated with radio-chemotherapy: correlation between length of treatment interruption and outcome. Int J Colorectal Dis. 2009;24:1421–1428.
- Sebag-Montefiore D, Meadows HM, Cunningham D, et al. Three cytotoxic drugs combined with pelvic radiation and as maintenance chemotherapy for patients with squamous cell carcinoma of the anus (SCCA): long-term follow-up of a phase II pilot study using 5-fluorouracil, mitomycin C and cisplatin. Radiother Oncol. 2012;104:155–160.
- Call JA, Prendergast BM, Jensen LG, et al. Intensity-modulated radiation therapy for anal cancer: results from a Multi-Institutional Retrospective Cohort Study. Am J Clin Oncol. 2016;39:8–12.
- Kapoor R, Khosla D, Shukla AK, et al. Dosimetric and clinical outcome in image-based high-dose-rate interstitial brachytherapy for anal cancer. Brachytherapy. 2014;13:388–393.
- Mai SK, Grieger J, Lachmann R, et al. Radiochemotherapy for anal carcinoma - effectivity and late toxicity. Onkologie. 2002;25:55–59.
- Konski A Jr, John M, Krieg R, et al. Evaluation of planned treatment breaks during radiation therapy for anal cancer: update of RTOG 92-08. Int J Radiat Oncol Biol Phys. 2008;72:114–118.
- Fraunholz IB, Haberl A, Klauke S, et al. Long-term effects of chemoradiotherapy for anal cancer in patients with HIV infection: oncological outcomes, immunological status, and the clinical course of the HIV disease. Dis Colon Rectum. 2014;57:423–431.
- Bieri S, Allal A, Kurtz J. Sphincter-conserving treatment of carcinomas of the anal margin. Acta Oncol. 2001;40:29–33.
- White EC, Khodayari B, Erickson KT, et al. Comparison of toxicity and treatment outcomes in HIV-positive versus HIV-negative patients with squamous cell carcinoma of the anal canal. Am J Clin Oncol. 2014;40:386–392.
- Gunderson LL, Winter KA, Ajani JA, et al. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol. 2012;30:4344–4351.
- Dewas CV, Maingon P, Dalban C, et al. Does gap-free intensity modulated chemoradiation therapy provide a greater clinical benefit than 3D conformal chemoradiation in patients with anal cancer? Radiat Oncol. 2012;7:201–210.
- Vordermark D, Sailer M, Flentje M, et al. Curative-intent radiation therapy in anal carcinoma: quality of life and sphincter function. Radiother Oncol. 1999;52:239–243.
- Tveit KM, Karlsen KO, Fosså SD, et al. Primary treatment of carcinoma of the anus by combined radiotherapy and chemotherapy primary treatment of carcinoma of the anus by combined radiotherapy and chemotherapy. Scand J Gastroenterol. 1989;24:1243–1247.
- John M, Flam M, Lovalvo L, et al. Feasibility of non-surgical definitive management of anal canal carcinoma. Int J Radiat Oncol Biol Phys. 1987;13:299–303.
- Tanum G, Tveit K, Karlsen K. Chemoradiotherapy of anal carcinoma: tumour response and acute toxicity. Oncology. 1993;50:14–17.
- Gabriele A, Rovea P, Sola B, et al. Radiation therapy and chemotherapy in the conservative treatment of carcinoma of the anal canal: survival and late morbidity in a series of 25 patients. Anticancer Res. 1997;17:653–656.
- Ortholan C, Ramaioli A, Peiffert D, et al. Anal canal carcinoma: early-stage tumors ≤10 mm (T1 or Tis): therapeutic options and original pattern of local failure after radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:479–485.
- Balamucki C, Zlotecki R, Rout W, et al. Squamous cell carcinoma of the anal margin: the university of Florida experience. Am J Clin Oncol. 2011;34:406–410.
- De Bari B, Jumeau R, Bouchaab H, et al. Efficacy and safety of helical tomotherapy with daily image guidance in anal canal cancer patients. Acta Oncol. 2016;55:767–773.
- Oblak I, Petric P, Anderluh F, et al. Anal cancer chemoirradiation with curative intent - a single institution experience. Neoplasma. 2009;56:150–155.
- Peiffert D, Bey P, Pernot M, et al. Conservative treatment by irradiation of epidermoid cancers of the anal canal: prognostic factors of tumoral control and complications . Int J Radiat Oncol Biol Phys. 1997;37:313–324.
- Nguyen NP, Vock J, Sroka T, et al. Feasibility of image-guided radiotherapy based on tomotherapy for the treatment of locally advanced anal carcinoma. Anticancer Res. 2011;31:4393–4396.
- Di Santo S, Trignani M, Neri M, et al. Radiochemotherapy in anal cancer: cCR, clinical outcomes and quality of life using two different treatment schedules. Reports Pract Oncol Radiother. 2015;20:128–134.
- Oblak I, Petric P, Anderluh F, et al. Long term outcome after combined modality treatment for anal cancer. Radiol Oncol. 2012;46:145–152.
- Jones RD, Symonds RP, Robertson AG, et al. Changes in the radiation treatment of cancer of the anus in Glasgow. Br J Radiol. 1993;66:797–800.
- Schlienger M, Krzisch C, Pene F, et al. Epidermoid carcinoma of the anal canal treatment results and prognostic variables in a series of 242 cases. Int J Radiat Oncol Biol Phys. 1989;17:1141–1151.
- Beck D, Karulf R. Combination therapy for epidermoid carcinoma of the anal canal. Dis Colon Rectum. 1994;37:1118–1125.
- Allal A, Kurtz J, Pipard G, et al. Chemoradiotherapy versus radiotherapy alone for anal cancer: a retrospective comparison. Int J Radiat Oncol Biol Phys. 1993;27:59–66.
- Cordoba A, Escande A, Leroy T, et al. Low-dose-rate interstitial brachytherapy boost for the treatment of anal canal cancers. Brachytherapy. 2017;16:230.
- Sandhu APS, Symonds RP, Robertson AG, et al. Interstitial iridium-192 implantation combined with external radiotherapy in anal cancer: ten years experience. Int J Radiat Oncol Biol Phys. 1998;40:575–581.
- Widder J, Kastenberger R, Fercher E, et al. Radiation dose associated with local control in advanced anal cancer: retrospective analysis of 129 patients. Radiother Oncol. 2008;87:367–375.
- Eng C, Chang GJ, You YN, et al. Long-term results of weekly/daily cisplatin-based chemoradiation for locally advanced squamous cell carcinoma of the anal canal. Cancer. 2013;119:3769–3775.
- Vaizey CJ, Carapeti E, Cahill JA, et al. Prospective comparison of faecal incontinence grading systems. Gut. 1999;44:77–80.
- Broens P, Van Limbergen E, Penninckx F, et al. Clinical and manometric effects of combined external beam irradiation and brachytherapy for anal cancer. Int J Colorectal Dis. 1998;13:68–72.
- Vordermark D, Flentje M, Sailer M, et al. Intracavitary afterloading boost in anal canal carcinoma. Results, function and quality of life. Strahlenther Onkol. 2001;177:252–258.
- Bentzen AG, Guren MG, Vonen B, et al. Faecal incontinence after chemoradiotherapy in anal cancer survivors: long-term results of a national cohort. Radiother Oncol. 2013;108:55–60.
- De Francesco I, Thomas K, Wedlake L, et al. Intensity-modulated radiotherapy and anal cancer: clinical outcome and late toxicity assessment. Clin Oncol. 2016;28:604–610.
- Vieillot S, Fenoglietto P, Lemanski C, et al. IMRT for locally advanced anal cancer: clinical experience of the Montpellier Cancer Center. Radiat Oncol. 2012;7:45.
- Mitchell MP, Abboud M, Eng C, et al. Intensity-modulated radiation therapy with concurrent chemotherapy for anal cancer: outcomes and toxicity. Am J Clin Oncol. 2014;37:461–466.
- Fakhrian K, Sauer T, Klemm S, et al. Radiotherapy with or without chemotherapy in the treatment of anal cancer: 20-year experience from a single institute. Strahlenther Onkol. 2013;189:18–25.
- Lestrade L, De Bari B, Pommier P, et al. Role of brachytherapy in the treatment of cancers of the anal canal: long-term follow-up and multivariate analysis of a large monocentric retrospective series. Strahlenther Onkol. 2014;190:546–554.
- Koerber SA, Slynko A, Haefner MF, et al. Efficacy and toxicity of chemoradiation in patients with anal cancer–a retrospective analysis. Radiat Oncol. 2014;9:113.
- Badvie S, Andreyev HJN. Topical phenylephrine in the treatment of radiation-induced faecal incontinence. Clin Oncol (R Coll Radiol). 2005;17:122–126.
- Maeda Y, Høyer M, Lundby L, et al. Temporary sacral nerve stimulation for faecal incontinence following pelvic radiotherapy. Radiother Oncol. 2010;97:108–112.
- Oehler-Jänne C, Seifert B, Lütolf UM, et al. Clinical outcome after treatment with a brachytherapy boost versus external beam boost for anal carcinoma. Brachytherapy. 2007;6:218–226.
- Kapp KS, Geyer E, Gebhart FH, et al. Experience with split-course external beam irradiation +/- chemotherapy and integrated Ir-192 high-dose-rate brachytherapy in the treatment of primary carcinomas of the anal canal. Int J Radiat Oncol Biol Phys. 2001;49:997–1005.
- Peiffert D, Bey P, Pernot M, et al. Conservative treatment by irradiation of epidermoid carcinomas of the anal margin. Int J Radiat Oncol Biol Phys. 1997;39:57–66.
- Myerson RJ, Outlaw ED, Chang A, et al. Radiotherapy for epidermoid carcinoma of the anus: thirty years’ experience. Int J Radiat Oncol Biol Phys. 2009;75:428–435.
- Vuong T, Devic S, Belliveau P, et al. Contribution of conformal therapy in the treatment of anal canal carcinoma with combined chemotherapy and radiotherapy: results of a phase II study. Int J Radiat Oncol Biol Phys. 2003;56:823–831.
- Edelman S, Johnstone PAS. Combined modality therapy for HIV-infected patients with squamous cell carcinoma of the anus: Outcomes and toxicities. Int J Radiat Oncol Biol Phys. 2006;66:206–211.
- Bosset JF, Roelofsen F, Morgan DAL, et al. Shortened irradiation scheme, continuous infusion of 5-fluorouracil and fractionation of mitomycin C in locally advanced anal carcinomas. Results of a phase II study of the European Organization for Research and Treatment of Cancer. Radiotherapy and Gastr. Eur J Cancer.2003;39:45–51.
- Flam M, John M, Mowry P, et al. Definitive combined modality therapy of carcinoma of the anus. A report of 30 cases including results of salvage therapy in patients with residual. Dis Colon Rectum. 1987;30:495–502.
- Flam M, John M, Lovalvo L, et al. Definitive nonsurgical therapy of epithelial malignancies of the anal canal. A report of 12 cases. Cancer. 1983;51:1378–1387.
- Doyen J, Benezery K, Follana P, et al. Predictive factors for early and late local toxicities in anal cancer treated by radiotherapy in combination with or without chemotherapy. Dis Colon Rectum. 2013;56:1125–1133.
- Oehler-Jänne C, Seifert B, Lutolf UM, et al. Local tumor control and toxicity in HIV-associated anal carcinoma treated with radiotherapy in the era of antiretroviral therapy. Radiat Oncol. 2006;1:29.
- Fraunholz I, Weiss C, Eberlein K, et al. Concurrent chemoradiotherapy with 5-fluorouracil and mitomycin C for invasive anal carcinoma in human immunodeficiency virus-positive patients receiving highly active antiretroviral therapy. Int J Radiat Oncol Biol Phys. 2010;76:1425–1432.
- Touboul E, Schlienger M, Buffat L, et al. Epidermoid carcinoma of the anal canal. Results of curative-intent radiation therapy in a series of 270 patients. Cancer. 1994;73:1569–1579.
- Tanum G, Tveit K, Karlsen KO, et al. Chemotherapy and radiation therapy for anal carcinoma. Survival and late morbidity. Cancer. 1991;67:2462–2466.
- Schneider IH, Grabenbauer GG, Reck T, et al. Combined radiation and chemotherapy for epidermoid carcinoma of the anal canal. Int J Colorectal Dis. 1992;7:192–196.
- Serkies K, Bednaruk-Mlynski E, Dziadziuszko R, et al. Conservative treatment for carcinoma of the anus-a report of 35 patients. Neoplasma. 2003;50:152–158.
- Hwang JM, Rao AR, Cosmatos HA, et al. Treatment of T3 and T4 anal carcinoma with combined chemoradiation and interstitial 192Ir implantation: a 10-year experience. Brachytherapy. 2004;3:95–100.
- Raffetto N, Monaco A, Banelli E. Radiotherapy and chemotherapy in the conservative treatment of anal canal carcinoma. Anticancer Res. 2008;28:1335–1339.
- Allal AS, Obradovic M, Laurencet F, et al. Treatment of anal carcinoma in the elderly: feasibility and outcome of radical radiotherapy with or without concomitant chemotherapy. Cancer. 1999;85:26–31.
- Tomaszewski JM, Link E, Leong T, et al. Twenty-five-year experience with radical chemoradiation for anal cancer. Int J Radiat Oncol Biol Phys. 2012;83:552–558.
- Peiffert D, Tournier-Rangeard L, Gérard JP, et al. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: final analysis of the randomized UNICANCER ACCORD 03 trial. J Clin Oncol. 2012;30:1941–1948.
- Oehler-Jänne C, Huguet F, Provencher S, et al. HIV-specific differences in outcome of squamous cell carcinoma of the anal canal: a multicentric cohort study of HIV-positive patients receiving highly active antiretroviral therapy. J Clin Oncol. 2008;26:2550–2557.
- Holland JM, Swift PS. Tolerance of patients with human immunodeficiency virus and anal carcinoma to treatment with combined chemotherapy and radiation therapy. Radiology. 1994;193:251–254.
- Hammad N, Heilbrun L, Gupta S, et al. Squamous cell cancer of the anal canal in HIV-infected patients receiving highly active antiretroviral therapy: a single institution experience. Am J Clin Oncol. 2011;34:135–139.
- Jephcott CR, Paltiel C, Hay J. Quality of life after non-surgical treatment of anal carcinoma: a case control study of long-term survivors. Clin Oncol (R Coll Radiol). 2004;16:530–535.
- Han K, Cummings BJ, Lindsay P, et al. Prospective evaluation of acute toxicity and quality of life after IMRT and concurrent chemotherapy for anal canal and perianal cancer. Int J Radiat Oncol Biol Phys. 2014;90:587–594.
- Tournier-rangeard L, Mercier M, Peiffert D, et al. Radiochemotherapy of locally advanced anal canal carcinoma: prospective assessment of early impact on the quality of life (randomized trial ACCORD 03) Radiother Oncol. 2008;87:391–397.
- Zachariah B, Gwede CK, James J, et al. Octreotide acetate in prevention of chemoradiation-induced diarrhea in anorectal cancer: randomized RTOG trial 0315. J Natl Cancer Inst. 2010;102:547–556.
- Oehler C, Provencher S, Donath D, et al. Chemo-radiation with or without mandatory split in anal carcinoma: experiences of two institutions and review of the literature. Radiat Oncol. 2010;5:36.
- Grabenbauer GG, Schneider IH, Gall FP, et al. Epidermoid carcinoma of the anal canal: treatment by combined radiation and chemotherapy. Radiother Oncol. 1993;27:59–62.
- Deo S, Shukla N, Raina V, et al. Organ-preserving multimodality management of squamous cell carcinoma of anal canal. Indian J Gastroenterol. 2005;24:210–214.
- Zampino MG, Magni E, Leonardi MC, et al. Concurrent cisplatin, continuous infusion fluorouracil and radiotherapy followed by tailored consolidation treatment in non metastatic anal squamous cell carcinoma. BMC Cancer. 2011;11:55.
- Rabbani AN, Zlotecki RA, Kirwan J, et al. Definitive radiotherapy for squamous cell carcinoma of the anal canal. Am J Clin Oncol. 2010;33:47–51.
- Mitra D, Hong TS, Horick N, et al. Long-term outcomes and toxicities of a large cohort of anal cancer patients treated with dose-painted IMRT per RTOG 0529. Adv Radiat Oncol. 2017;2:110–117.
- Saarilahti K, Arponen P, Vaalavirta L, et al. The effect of intensity-modulated radiotherapy and high dose rate brachytherapy on acute and late radiotherapy-related adverse events following chemoradiotherapy of anal cancer. Radiother Oncol. 2008;87:383–390.
- Pironi D, Panarese A, Vendettuoli M, et al. Chronic radiation-induced proctitis: the 4% formalin application as non-surgical treatment. Int J Colorectal Dis. 2013;28:261–266.
- De Parades V, Etienney I, Bauer P, et al. Formalin application in the treatment of chronic radiation-induced hemorrhagic proctitis - an effective but not risk-free procedure: a prospective study of 33 patients. Dis Colon Rectum. 2005;48:1535–1541.
- Rich TA, Ajani JA, Morrison WH, et al. Chemoradiation therapy for anal cancer: radiation plus continuous infusion of 5-fluorouracil with or without cisplatin. Radiother Oncol. 1993;27:209–215.
- Glover M, Smerdon GR, Andreyev HJ, et al. Hyperbaric oxygen for patients with chronic bowel dysfunction after pelvic radiotherapy (HOT2): a randomised, double-blind, sham-controlled phase 3 trial. Lancet Oncol. 2016;17:224–233.
- Khanfir K, Ozsahin M, Bieri S, et al. Patterns of failure and outcome in patients with carcinoma of the anal margin. Ann Surg Oncol. 2008;15:1092–1098.
- Cantril S, Green J, Schall G, et al. Primary radiation therapy in the treatment of anal carcinoma. Int J Radiat Oncol Biol Phys. 1983;9:1271–1278.
- Myerson R, Shapiro S, Lacey D, et al. Carcinoma of the anal canal. Am J Clin Oncol. 1995;18:32–39.
- Newman G, Calverley DC, Acker BD, et al. The management of carcinoma of the anal canal by external beam radiotherapy, experience in Vancouver 1971-1988. Radiother Oncol. 1992;25:196–202.
- Stojanović S, Radošević-Jelic L, Dabić-Stankovic K, et al. External beam radiotherapy plus brachytherapy boost in treatment of anal canal carcinoma. Acta Chir Iugosl. 2007;54:33–38.
- Wexler A, Berson AM, Goldstone SE, et al. Invasive anal squamous-cell carcinoma in the HIV-positive patient: Outcome in the era of highly active antiretroviral therapy. Dis Colon Rectum. 2008;51:73–81.
- Fallai C, Cerrotta A, Valvo F, et al. Anal carcinoma of the elderly treated with radiotherapy alone or with concomitant radio-chemotherapy. Crit Rev Oncol Hematol. 2007;61:261–268.
- Chadha M, Rosenblatt E. a, Malamud S, et al. Squamous-cell carcinoma of the anus in HIV-positive patients. Dis Colon Rectum. 1994;37:861–865.
- Wolff H, Raus I, Jung K, et al. High-grade acute organ toxicity as a positive prognostic factor in primary radiochemotherapy for anal carcinoma. Int J Radiat Oncol Biol Phys. 2011;79:1467–1478.
- Doci R, Zucali R, La MG, et al. Primary chemoradiation therapy with fluorouracil and cisplatin for cancer of the anus: results in 35 consecutive patients. J Clin Oncol. 1996;14:3121–3125.
- Young SC, Solomon MJ, Hruby G, et al. Review of 120 anal cancer patients. Colorectal Dis. 2009;11:909–914.
- Falk A, Claren A, Benezery K, et al. Interstitial high-dose rate brachytherapy as boost for anal canal cancer. Radiat Oncol. 2014;9:240.
- Doci R, Zucali R, Bombelli L, et al. Combined chemoradiation therapy for anal cancer. A report of 56 cases. Ann Surg. 1992;215:150–156.
- Wagner J, Mahe MA, Romestaing P, et al. Radiation therapy in the conservative treatment of carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 1994;29:17–23.
- Barriger BR, Calley C, Cárdenes HR. Treatment of anal carcinoma in immune-compromised patients. Clin Transl Oncol. 2009;11:609–614.
- Gerard JP, Ayzac L, Hun D, et al. Treatment of anal canal carcinoma with high dose radiation therapy and concomitant fluorouracil-cisplatinum. Long-term results in 95 patients. Radiother Oncol. 1998;46:249–256.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376.
- Joseph K, Vos LJ, Warkentin H, et al. Patient reported quality of life after helical IMRT based concurrent chemoradiation of locally advanced anal cancer. Radiother Oncol.2016;120:228–233.
- Allal AS, Sprangers M, Laurencet F, et al. Assessment of long-term quality of life in patients with anal carcinomas treated by radiotherapy with or without chemotherapy. Br J Cancer. 1999;80:1588–1594.
- Sparano JA, Lee JY, Palefsky J, et al. Cetuximab plus chemoradiotherapy for HIV-associated anal carcinoma: a phase II AIDS malignancy consortium trial. J Clin Oncol. 2017;35:727–733.
- Gujral S, Conroy T, Fleissner C, et al. Assessing quality of life in patients with colorectal cancer: an update of the EORTC quality of life questionnaire. Eur J Cancer. 2007;43:1564–1573.
- Eypasch E, Williams J, Wood-Dauphinee S, et al. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg. 1995;82:216–222.
- Provencher S, Oehler C, Lavertu S, et al. Quality of life and tumor control after short split-course chemoradiation for anal canal carcinoma. Radiat Oncol. 2010;5:41–49.
- Welzel G, Hägele V, Wenz F, et al. Quality of life outcomes in patients with anal cancer after combined radiochemotherapy. Strahlenther Onkol. 2011;187:175–182.
- Milano MT, Jani AB, Farrey KJ, et al. Intensity-modulated radiation therapy (IMRT) in the treatment of anal cancer: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. 2005;63:354–361.
- Salama JK, Mell LK, Schomas DA, et al. Concurrent chemotherapy and intensity-modulated radiation therapy for anal canal cancer patients: a multicenter experience. J Clin Oncol. 2007;25:4581–4586.
- Franco P, Mistrangelo M, Arcadipane F, et al. Intensity-modulated radiation therapy with simultaneous integrated boost combined with concurrent chemotherapy for the treatment of anal cancer patients : 4-year results of a consecutive case series. Cancer Invest. 2015;33:259–266.
- Tozzi A, Cozzi L, Iftode C, et al. Radiation therapy of anal canal cancer: from conformal therapy to volumetric modulated arc therapy. BMC Cancer. 2014;14:833–842.
- Tagliaferri L, Manfrida S, Barbaro B, et al. MITHRA - Multiparametric MR/CT image adapted brachytherapy (MR/CT-IABT) in anal canal cancer: a feasibility study. J Contemp Brachytheraphy. 2015;5:336–345.
- Kachnic LA, Tsai HK, Coen JJ, et al. Dose-painted intensity-modulated radiation therapy for anal cancer: a multi-institutional report of acute toxicity and response to therapy. Int J Radiat Oncol Biol Phys. 2012;82:153–158.
- Devisetty K, Mell LK, Salama JK, et al. A multi-institutional acute gastrointestinal toxicity analysis of anal cancer patients treated with concurrent intensity-modulated radiation therapy (IMRT) and chemotherapy. Radiother Oncol. 2009;93:298–301.
- Julie DAR, Oh JH, Apte AP, et al. Predictors of acute toxicities during definitive chemoradiation using intensity-modulated radiotherapy for anal squamous cell carcinoma. Acta Oncol (Madr). 2016;55:208–216.
- Engineer R, Mallik S, Mahantshetty U, et al. Impact of radiation dose on locoregional control and survival on squamous cell carcinoma of anal canal. Radiother Oncol. 2010;95:283–287.
- Stone HB, Coleman CN, Anscher MS, et al. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–536.
- Alsadius D, Hedelin M, Lundstedt D, et al. Mean absorbed dose to the anal-sphincter region and fecal leakage among irradiated prostate cancer survivors. Int J Radiat Oncol Biol Phys. 2012;84:e181.
- Peeters STH, Hoogeman MS, Heemsbergen WD, et al. Rectal bleeding, fecal incontinence, and high stool frequency after conformal radiotherapy for prostate cancer: normal tissue complication probability modeling. Int J Radiat Oncol Biol Phys. 2006;66:11–19.
- Heemsbergen WD, Hoogeman MS, Hart GA, et al. Gastrointestinal toxicity and its relation to dose distributions in the anorectal region of prostate cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1011–1018.
- Buettner F, Gulliford SL, Webb S, et al. The dose-response of the anal sphincter region-an analysis of data from the MRC RT01 trial. Radiother Oncol. 2012;103:347–352.
- Rubin EB, Buehler AE, Halpern SD. States worse than death among hospitalized patients with serious illnesses. JAMA Intern Med. 2016;176:1557–1559.
- Sodergren SC, Johnson CD, Gilbert A, et al. Phase I-III development of the EORTC QLQ-ANL27, a health-related quality of life questionnaire for anal cancer. Radiother Oncol. 2018;126:222–228.