Abstract
Introduction: Large anatomical variations can be observed during the treatment course intensity-modulated radiotherapy (IMRT) for head and neck cancer (HNC), leading to potential dose variations. Adaptive radiotherapy (ART) uses one or several replanning sessions to correct these variations and thus optimize the delivered dose distribution to the daily anatomy of the patient. This review, which is focused on ART in the HNC, aims to identify the various strategies of ART and to estimate the dosimetric and clinical benefits of these strategies.
Material and methods: We performed an electronic search of articles published in PubMed/MEDLINE and Science Direct from January 2005 to December 2016. Among a total of 134 articles assessed for eligibility, 29 articles were ultimately retained for the review. Eighteen studies evaluated dosimetric variations without ART, and 11 studies reported the benefits of ART.
Results: Eight in silico studies tested a number of replanning sessions, ranging from 1 to 6, aiming primarily to reduce the dose to the parotid glands. The optimal timing for replanning appears to be early during the first two weeks of treatment. Compared to standard IMRT, ART decreases the mean dose to the parotid gland from 0.6 to 6 Gy and the maximum dose to the spinal cord from 0.1 to 4 Gy while improving target coverage and homogeneity in most studies. Only five studies reported the clinical results of ART, and three of those studies included a non-randomized comparison with standard IMRT. These studies suggest a benefit of ART in regard to decreasing xerostomia, increasing quality of life, and increasing local control. Patients with the largest early anatomical and dose variations are the best candidates for ART.
Conclusion: ART may decrease toxicity and improve local control for locally advanced HNC. However, randomized trials are necessary to demonstrate the benefit of ART before using the technique in routine practice.
Introduction
Intensity-modulated radiotherapy (IMRT) is classically based on a single initial planning computed tomography (CT) scan, whereas large anatomical variations can be observed during the treatment course. In the case of head and neck IMRT, these variations are body weight loss, primary tumor shrinkage, parotid gland displacement, and volume reduction [Citation1–3]. Due to the steep IMRT dose gradient and the potential anatomic variations, the actual delivered dose may not correspond to the planned dose. The result can be an increase in the doses delivered to organs at risk (OAR) and/or a decrease in the doses delivered to the tumor, resulting in increased risk of toxicity and recurrence [Citation4,Citation5].
The standard approach for image-guided radiotherapy (IGRT) in head and neck cancer (HNC) consists of rigid registration based on the bony anatomy using planar or cone beam CT images. Daily IGRT allows a reduction of the setup errors to 1.5 mm [Citation6,Citation7]. However, the flexibility between rigid bony structures and deformations of the target volume and OAR may lead to different local misalignments, which are impossible to correct simultaneously with a single couch displacement [Citation8,Citation9]. Adaptive radiotherapy (ART) using one or several replanning sessions aims to correct these variations and thus optimize the delivered dose distribution to the anatomy of the patient. Based on daily CTs, several ART strategies can be designed that potentially require technical and organizational actions, such as dose monitoring based on the dose-per-fraction calculation, cumulated dose during treatment, and replanning. Since the use of ART is very new, its benefit can be estimated either in silico, using deformable image registration (DIR) for dose accumulation, or clinically, based on a limited number of studies. However, the choice of the optimal algorithm for dose accumulation is particularly complex. The following questions also arise when considering the clinical goal of ART: Do all patients need ART? Does ART aim to increase local control, decrease toxicity, or both? What is the optimal number and timing of replanning scans? Does the increase in the patient’s quality of life counterbalance the human and economic resources required throughout the process? Several studies have tried to solve these questions. It has now been well established that only a subset of patients may benefit from ART. However, the other questions remain open.
This review focused on ART in the head and neck aimed to (i) identify the various strategies of ART and (ii) estimate the dosimetric and clinical benefits of these strategies. In the first part of the review, the dosimetric benefit of ART for sparing OAR and increasing the dose to the target volume was reviewed. In the second part of the review, the clinical results of ART were analyzed. Finally, the methodological aspects and the limitations of these studies were discussed, as well as the identification of the subgroup of patients who are more likely to benefit from ART.
Material and methods
We performed an electronic search of articles published in PubMed/MEDLINE and Science Direct from January 2005 to December 2016. The search was performed according to PRISMA guidelines [Citation10] using the following keywords: ART, anatomical and/or functional and/or biomarkers variations, HNC, parotid glands, and target volume. Our search was restricted to English articles dealing with locally advanced HNC and anatomical/dosimetric variation in the context of ART. In addition, the references of each study were screened to retrieve additional relevant papers. All included studies were reviewed to collect the following data: study design (prospective vs. retrospective), number of patients, diagnosis of the included patients, time and modality of evaluation, anatomical and/or dosimetric variations, and dosimetric and/or clinical benefit of ART. Retrospective studies with a limited number of patients (<15 patients) were excluded to avoid bias.
Results
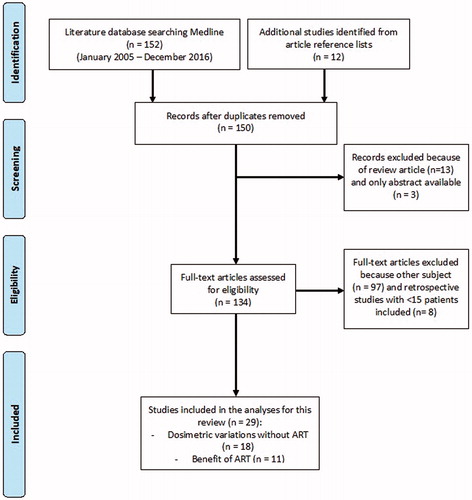
The PRISMA flow diagram of the literature search is shown in . One hundred fifty-two studies were identified from PubMed/MEDLINE. Another twelve studies were identified from reference lists. Three studies were excluded because only the abstract was available, and thirteen studies were excluded because they were general reviews. One hundred thirty-four articles were assessed for eligibility. Ninety-seven studies did not meet the eligibility criteria, as most of them were focused on dose calculation or algorithms for image registration. Eight supplementary studies were excluded due to their retrospective nature with a limited number of patients (<15).
Finally, a total of 29 studies were included in the analyses for this review. Eighteen studies reported dosimetric variations without ART (Supplementary Tables S1 and S2), and 11 studies reported the benefits of ART, providing either dosimetric data () or clinical results (). These studies included between 10 and 86 patients, who were treated or simulated with ART.
Table 1. Dosimetric benefits of ART in patients with head and neck cancer.
Table 2. Clinical benefits of ART in patients with head and neck cancer.
Strategies of ART
The number of replanning sessions ranged from one (72% of the studies) to six (10% of the studies), which were performed in most cases during the 3rd or 4th week of treatment. For comparisons between ART and no-ART, two types of approaches were used, either by considering another cohort of non-ART patients or by using a single group of patients undergoing treatment simulation with or without ART. The dose comparison could be performed based on the fraction or by considering the cumulated dose calculated based on the mean dose or using DIR ().
Dosimetric benefits of ART
A total of eight studies reported data on the dosimetric benefits of ART: eight studies addressed sparing the parotid gland, three studies addressed the spinal cord and five studies investigated improving the target volume coverage ().
Parotid glands
The difference between the mean planned doses and the mean delivered doses without ART ranged in the literature from –1 Gy to 6 Gy, with 85% of studies showing an increase in the parotid gland dose (Supplementary Table S1) [Citation2,Citation12,Citation14,Citation21–30]. Moreover, the increase in the parotid gland dose appeared to be strongly patient-dependent and was observed only in 30 to 65% of patients. Furthermore, a small proportion of patients (10%-30%) were reported to present a spontaneous decrease in the parotid gland dose [Citation12,Citation25].
Compared with the mean dose delivered with standard IMRT, ART allowed a dose reduction ranging from 0.6 Gy to 4.1 Gy in all studies [Citation11–15,Citation17,Citation31] except one, which did not find a benefit with one replanning session [Citation3] (). Surprisingly, the parotid glands with a dose decrease without ART have a benefit of ART, which allows further reducing the mean dose by 1.4 Gy [Citation12]. The benefit of ART appears to correlate with the number of replanning sessions [Citation32]. The dosimetric benefit of numerous replanning strategies (63 combinations), defined by various numbers (one to six), and the timing of replanning (during each week of the treatment), was investigated in 13 patients with oropharyngeal cancers [Citation33]. Six weekly replanning sessions ensured the best benefits, allowing a decrease in the mean dose of 3.3 Gy. However, 94% of this benefit was already attained with three replanning sessions at weeks one, two, and five, enabling a reduction of the mean dose by 3.1 Gy.
Spinal cord
Eleven studies reported dose variations for the spinal cord [Citation2,Citation11,Citation21–24,Citation27–29,Citation34,Citation35]. Compared to planning and treatment without ART, the maximum dose to the spinal cord increased from –0.1 Gy to 3.8 Gy (Supplementary Table S1), depending on the patient. However, in most cases, the maximum dose did not increase higher than the initial spinal cord dose constraints in the IMRT planning (<45–48 Gy) [Citation34]. This dose increase correlated with positional variability but not with anatomical variation [Citation21].
Three studies reported that ART achieved a dosimetric benefit for the spinal cord, decreasing the spinal cord maximum dose by 0.1 Gy to 4 Gy for several fractions, ranging from one to all fractions [Citation3,Citation11,Citation13]. The benefit of this dosimetric difference is unclear and may not translate into a clinical benefit.
Target volume (GTV, CTV, and PTV)
The analysis of the differences between planned and delivered doses without ART for the gross total volume (GTV), clinical target volume (CTV), and planning target volume (PTV) showed controversial results. While most studies found very low dose differences (below 1–2% for the D2% and D95%), some studies reported large dose differences [Citation21,Citation34,Citation36], such as a reduction of the dose to the GTV by 1.9 Gy [Citation21] or a mean reduction of the minimum dose to the PTV by 2 Gy [Citation34] (Supplementary Table S2).
Five studies reported the dosimetric benefit of ART in increasing the dose to the target volume. All of these studies reported various improved dosimetric endpoints (for GTV, CTV, and PTV) with more uniform coverage [Citation13,Citation15,Citation31] and increased coverage [Citation14,Citation31,Citation37], such as an increase of 2.1 Gy in the dose to the PTV (D95%) [Citation37] ().
Clinical benefits of ART
Only five studies reported the clinical benefits of ART (). The number of patients per study ranged from 22 to 317, with a total number of 603 patients. The median follow-up ranged from 29 to 38 months. Tumors were located primarily in the oropharynx [Citation16,Citation18,Citation38] or the nasopharynx [Citation17,Citation19]. The dose delivered to the high-risk volume ranged from 66 to 76 Gy. In all studies, the patients were treated with definitive chemoradiotherapy, while one study [Citation20] also included patients treated with surgery followed by radiotherapy. A 3–5 mm 3D margin around the CTV was used to generate the PTV. Replanning was performed either at a specific time point in two studies [Citation18,Citation19] or based on the decision of the radiation oncologist [Citation16,Citation17,Citation20]. A large majority of patients (197 patients) had only one replanning session, while 31 patients had two replanning sessions. The time point ranged from the second fraction to the 28th fraction for the first replanning session and from the 11th fraction to the 25th fraction for the second replanning session. Three studies reported the clinical results of ART in comparison with a no-ART technique [Citation17,Citation19,Citation38] without randomization. Two studies were prospective [Citation16,Citation19], while the remaining three studies were retrospective [Citation17,Citation18,Citation20].
Survival and loco-regional control
The 2-year loco-regional control rates (LRCs) for patients treated with ART ranged from 88% to 97% [Citation16,Citation19,Citation20], compared to the 80% rate that was previously reported in the literature [Citation39–41]. The 2-year LRC rate was significantly improved for the patients with replanning in comparison to the patients without replanning in two studies (97.2% and 88% for ART compared to 82.2% and 79% for no-ART) [Citation19,Citation20]. In a study performed by Zhao et al. [Citation17], ART significantly increased the 3-year loco-regional free survival for patients with stage T3-T4 only (80% for ART patients, compared to 60% for patients without ART (p = .03) [Citation17]), while no difference was found for early-stage disease (T1-T2) or in the case of large lymph node volumes (N2, N3).
Three studies reported a 2-year overall survival ranging from 73% to 90% [Citation18–20]. These results were comparable with those reported in the literature for patients treated without ART [Citation39–41]. Indeed, the two studies with a comparison arm did not show a significant difference between the two treatments (ART and no-ART) [Citation19,Citation20].
Toxicities and quality of life
Acute and late toxicity were available for the five studies () and were comparable to the toxicity rates that were previously published for patients treated without ART [Citation39,Citation42–45]. In a study performed by Schwartz et al. [Citation16,Citation31], the quality of life score at 20 months demonstrated full preservation or the functional recovery of speech and eating. However, as stated by the authors, these results were preliminary and must be validated in a larger cohort of patients. Regarding the three studies with a comparison arm, there was no difference in late toxicity between ART and no-ART in two studies [Citation17,Citation20]. Only one study that included 129 patients (86 patients underwent ART and 43 patients refused the replanning) with stage I-IV nasopharyngeal carcinoma showed an improvement in quality of life with ART in comparison to no-ART [Citation19]. The score from EORTC QLQ-C30 [Citation46] for global quality of life 12 months after treatment was 82 for ART in comparison to 78 for no-ART (p = .01). Regarding specific symptoms, significant differences were also observed in role functioning, social functioning, dyspnea, appetite loss, constipation, diarrhea, speech problems, trouble with social contact, teeth, opening the mouth, dry mouth, and sticky saliva.
Discussion
For patients with locally advanced HNC, a weekly replanning session [Citation32] or at least early replanning [Citation25,Citation33,Citation47] appears to be the optimal ART strategy for sparing the parotid gland. ART appears to be justified for avoiding a parotid gland overdose [Citation12,Citation14] and therefore decreasing the risk of xerostomia [Citation16]. For the spinal cord, positional variation was considered to be the main cause of dose variation, and no strong evidence for the benefit of replanning was shown. The benefit of ART for other OARs (submandibular glands, oral cavity) is unclear due to the lack of data. ART appeared to correct target volume underdosing for a subset of patients [Citation11,Citation14,Citation15] and improved local control in non-randomized studies [Citation17,Citation19,Citation38].
Overall, these non-randomized and mostly retrospective studies show that ART is safe and may increase local control and to a lesser extent, improve quality of life by decreasing toxicity (xerostomia). Therefore, the level of evidence for the clinical benefit of ART over no-ART is weak.
The methods used to evaluate the dosimetric benefit of ART vary according to the type of per-treatment imaging, imaging frequency, methods used to evaluate the dose and dosimetric endpoints (). A limited number of patients were included in each study (from 10 to 33 patients), decreasing the statistical power of the results. Tumor localization may also impact the results (oropharynx or nasopharynx). Moreover, per-treatment imaging was performed at different time points and frequencies during IMRT, from only once to each week. Only three studies used more than two time points to estimate the cumulated dose. Moreover, the dosimetric impact of anatomical variations was estimated using different dosimetric endpoints. Indeed, most of the studies reported variations in the mean dose (in Gy or in % of the prescribed dose), which have been shown to be predictive of xerostomia [Citation48]. A few studies also used the normal tissue complication probability (NTCP) model to estimate the increase in the xerostomia risk (n = 1, m = 0.4, and the median toxic dose ([TD50] = 39.9 [Citation49,Citation50]).
The dose comparison could be performed at the fraction [Citation3] or considering the cumulated dose, calculated based on the mean dose for a subset of fractions [Citation13,Citation17] or for the whole treatment [Citation11]. Dose accumulation could otherwise be estimated using DIR [Citation12,Citation14,Citation15,Citation31]. The main limitation of the DIR approach is the accuracy of the elastic registration algorithm, which is linked to the uncertainty of point-to-point correspondence between the planning CT and daily imaging. Several methods were therefore described to evaluate the accuracy of DIR algorithms, such as visual evaluation [Citation51], similarity indices (Dice similarity, for example) [Citation52], point-to-point error [Citation41] or phantom [Citation53,Citation54]. In practice, DIR can be used routinely for contour propagation since the contours can be visually validated/corrected by radiation oncologists [Citation55,Citation56]. However, the analysis of the literature shows that DIR is not yet fully validated in routine practice for dose accumulation. Recommendations for quality assurance and quality control for image registration in radiotherapy were recently published by the American Association of Physicists in Medicines (Radiation Therapy Committee Task Group No. 132) [Citation57]. Thereby, due to the variability between the different studies (number of time points and methods used to cumulate the dose, accuracy of the DIR methods, doses reported in Gy or in %), it appears to be difficult to estimate the true benefit of ART for sparing the parotid gland and decreasing xerostomia.
In this context, studies that provide clinical endpoints should be more relevant than in silico studies. Only five studies [Citation16–19,Citation38] reported clinical endpoints. However, these studies were either retrospective [Citation17,Citation18,Citation38] or non-randomized [Citation16,Citation19], and replanning was performed based on the radiation oncologist’s decision. As the patients with major tumor shrinkage underwent replanning more often than patients without a tumor response, the potential advantage of ART exhibited in these studies may be due to the selection of tumors with a better prognosis. Regarding toxicities, one study reported an improvement in the quality of life for patients treated with ART [Citation19]. However, due to the lack of randomization, the benefit of ART for reducing toxicity is unclear due to potential confounding factors (better tumor response, differences at baseline, replanning at different times and differences in tumor localization).
ART is particularly time-consuming [Citation17,Citation37], requires many resources, is expensive and carries the risk of error, thus requiring rigorous quality-assurance procedures. Dosimetric and clinical results have demonstrated that ART may not be useful for all patients. It is therefore crucial to target patients with OAR overdose and/or target volume underdose. Systematic weekly dose surveillance based on CBCT resulted in an increased workload (100 min per patients) for a limited parotid gland dose decrease (<1 Gy) among a limited number of patients [Citation58]. Ideally, replanning decisions should be based on early and simple anatomical predictors, with a clear objective of parotid gland overdose and/or tumor underdose correction. Good candidates for ART are likely patients with bulky tumors, early tumor shrinkage or early parotid gland overdose [Citation47,Citation59,Citation60]. Several limitations remain concerning the issue of patient selection: the limited number of patients in the studies [Citation47], the small number of per-treatment imaging sessions [Citation60], the unclear criteria for performing replanning [Citation59], and the lack of external validation of the models.
In addition to sparing OARs, ART may be useful for increasing local control via dose escalation. Based on the assumption that 18-FDG-avid regions of the tumor are radioresistant and may be at the origin of the recurrence [Citation61,Citation62], PET can be used to guide dose escalation [Citation63,Citation64]. Due to the anatomical and biological variations of the target volume during treatment [Citation65], anatomical and metabolic ART should be considered to maintain plan quality [Citation66]. However, only three prospective studies from the same treatment center (Ghent University Hospital, Belgium) addressed this complex strategy [Citation15,Citation23,Citation67]. This adaptive dose painting by numbers (A-DPBN) achieved higher 3-year local control (90%) than treatment with standard IMRT (79%). Regarding late toxicity, no difference was reported between A-DPBN and standard IMRT. Nevertheless, due to the weak robustness of the PET information and the potential variation during treatment, clinical studies, such as the ongoing NCT01341535 study (phase II randomized study comparing adaptive dose escalation to standard IMRT), are necessary to establish feasibility and to evaluate the benefits of A-DPBN.
In summary, due to the technique’s high cost, improved identification of the subset of patients who will experience reduced toxicity and/or improved local control after ART is required. The frequency and timing of replanning are not yet clearly defined. The use of IGRT images (CBCT or MRI) to monitor early anatomical and/or dosimetric variations seems to be the best solution to guide ART [Citation68,Citation69]. Indeed, early changes in CBCT were correlated with late xerostomia in a retrospective analysis of 119 patients [Citation68]. Dosimetric variations may also be estimated thanks to the use of electronic portal imaging device (EPID) [Citation70]. However, ongoing randomized trials are necessary to demonstrate the benefits of ART in comparison to no-ART before using the technique in routine practice in patients with locally advanced HNC. The new combined MRI/LINAC machines [Citation71] will allow acquiring real-time anatomical and functional data [Citation72,Citation73] and potentially to predict the treatment outcome [Citation74]. Thanks to these MRI/LINAC machines, the ideal ART strategy will be guided by real-time data correlated with treatment outcomes.
Supplemental Material
Download Zip (59.3 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Barker JL Jr., Garden AS, Ang KK, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys. 2004;59:960–970.
- Nishi T, Nishimura Y, Shibata T, et al. Volume and dosimetric changes and initial clinical experience of a two-step adaptive intensity modulated radiation therapy (IMRT) scheme for head and neck cancer. Radiother Oncol. 2013;106:85–89.
- Duma MN, Kampfer S, Schuster T, et al. Adaptive radiotherapy for soft tissue changes during helical tomotherapy for head and neck cancer. Strahlenther Onkol. 2012;188:243–247.
- Gregoire V, Jeraj R, Lee JA, et al. Radiotherapy for head and neck tumours in 2012 and beyond: conformal, tailored, and adaptive? Lancet Oncol. 2012;13:e292–e300.
- Schwartz DL. Current progress in adaptive radiation therapy for head and neck cancer. Curr Oncol Rep. 2012;14:139–147.
- Zeidan OA, Langen KM, Meeks SL, et al. Evaluation of image-guidance protocols in the treatment of head and neck cancers. Int J Radiat Oncol Biol Phys. 2007;67:670–677.
- Den RB, Doemer A, Kubicek G, et al. Daily image guidance with cone-beam computed tomography for head-and-neck cancer intensity-modulated radiotherapy: a prospective study. Int J Radiat Oncol Biol Phys. 2010;76:1353–1359.
- Djordjevic M, Sjoholm E, Tullgren O, et al. Assessment of residual setup errors for anatomical sub-structures in image-guided head-and-neck cancer radiotherapy. Acta Oncol. 2014;53:646–653.
- van Kranen S, van Beek S, Mencarelli A, et al. Correction strategies to manage deformations in head-and-neck radiotherapy. Radiother Oncol. 2010;94:199–205.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
- Capelle L, Mackenzie M, Field C, et al. Adaptive radiotherapy using helical tomotherapy for head and neck cancer in definitive and postoperative settings: initial results. Clin Oncol (R Coll Radiol). 2012;24:208–215.
- Castelli J, Simon A, Louvel G, et al. Impact of head and neck cancer adaptive radiotherapy to spare the parotid glands and decrease the risk of xerostomia. Radiat Oncol. 2015;10:6.
- Dewan A, Sharma S, Dewan A, et al. Impact of adaptive radiotherapy on locally advanced head and neck cancer - a dosimetric and volumetric study. Asian Pac J Cancer Prev. 2016;17:985–992.
- Jensen AD, Nill S, Huber PE, et al. A clinical concept for interfractional adaptive radiation therapy in the treatment of head and neck cancer. Int J Radiat Oncol Biol Phys. 2012;82:590–596.
- Olteanu LA, Berwouts D, Madani I, et al. Comparative dosimetry of three-phase adaptive and non-adaptive dose-painting IMRT for head-and-neck cancer. Radiother Oncol. 2014;111:348–353.
- Schwartz DL, Garden AS, Thomas J, et al. Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83:986–993.
- Zhao L, Wan Q, Zhou Y, et al. The role of replanning in fractionated intensity modulated radiotherapy for nasopharyngeal carcinoma. Radiother Oncol. 2011;98:23–27.
- Kataria T, Gupta D, Goyal S, et al. Clinical outcomes of adaptive radiotherapy in head and neck cancers. Br J Radiol. 2016;89:20160085.
- Yang H, Hu W, Wang W, et al. Replanning during intensity modulated radiation therapy improved quality of life in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2013;85:e47–e54.
- Chen AM, Daly ME, Cui J, et al. Clinical outcomes among patients with head and neck cancer treated by intensity-modulated radiotherapy with and without adaptive replanning. Head Neck. 2014;36:1541–1546.
- Ahn PH, Chen CC, Ahn AI, et al. Adaptive planning in intensity-modulated radiation therapy for head and neck cancers: single-institution experience and clinical implications. Int J Radiat Oncol Biol Phys. 2011;80:677–685.
- Beltran M, Ramos M, Rovira JJ, et al. Dose variations in tumor volumes and organs at risk during IMRT for head-and-neck cancer. J Appl Clin Med Phys. 2012;13:101–111.
- Berwouts D, Olteanu LA, Duprez F, et al. Three-phase adaptive dose-painting-by-numbers for head-and-neck cancer: initial results of the phase I clinical trial. Radiother Oncol. 2013;107:310–316.
- Ho KF, Marchant T, Moore C, et al. Monitoring dosimetric impact of weight loss with kilovoltage (kV) cone beam CT (CBCT) during parotid-sparing IMRT and concurrent chemotherapy. Int J Radiat Oncol Biol Phys. 2012;82:e375–e382.
- Hunter KU, Fernandes LL, Vineberg KA, et al. Parotid glands dose-effect relationships based on their actually delivered doses: implications for adaptive replanning in radiation therapy of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2013;87:676–682.
- Marzi S, Pinnarò P, D’Alessio D, et al. Anatomical and dose changes of gross tumour volume and parotid glands for head and neck cancer patients during intensity-modulated radiotherapy: effect on the probability of xerostomia incidence. Clin Oncol (R Coll Radiol). 2012;24:e54–e62.
- O’Daniel JC, Garden AS, Schwartz DL, et al. Parotid gland dose in intensity-modulated radiotherapy for head and neck cancer: is what you plan what you get? Int J Radiat Oncol Biol Phys. 2007;69:1290–1296.
- Orban de Xivry J, Castadot P, Janssens G, et al. Evaluation of the radiobiological impact of anatomic modifications during radiation therapy for head and neck cancer: can we simply summate the dose? Radiother Oncol. 2010;96:131–138.
- Robar JL, Day A, Clancey J, et al. Spatial and dosimetric variability of organs at risk in head-and-neck intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1121–1130.
- Yip C, Thomas C, Michaelidou A, et al. Co-registration of cone beam CT and planning CT in head and neck IMRT dose estimation: a feasible adaptive radiotherapy strategy. Br J Radiol. 2014;87:20130532.
- Schwartz DL, Garden AS, Shah SJ, et al. Adaptive radiotherapy for head and neck cancer-dosimetric results from a prospective clinical trial. Radiother Oncol. 2013;106:80–84.
- Wu Q, Chi Y, Chen PY, et al. Adaptive replanning strategies accounting for shrinkage in head and neck IMRT. Int J Radiat Oncol Biol Phys. 2009;75:924–932.
- Zhang P, Simon A, Rigaud B, et al. Optimal adaptive IMRT strategy to spare the parotid glands in oropharyngeal cancer. Radiother Oncol. 2016;120:41–47.
- Bhide SA, Davies M, Burke K, et al. Weekly volume and dosimetric changes during chemoradiotherapy with intensity-modulated radiation therapy for head and neck cancer: a prospective observational study. Int J Radiat Oncol Biol Phys. 2010;76:1360–1368.
- Height R, Khoo V, Lawford C, et al. The dosimetric consequences of anatomic changes in head and neck radiotherapy patients. J Med Imaging Radiat Oncol. 2010;54:497–504.
- Chen C, Fei Z, Chen L, et al. Will weight loss cause significant dosimetric changes of target volumes and organs at risk in nasopharyngeal carcinoma treated with intensity-modulated radiation therapy? Med Dosim. 2014;39:34–37.
- Hansen EK, Bucci MK, Quivey JM, et al. Repeat CT imaging and replanning during the course of IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;64:355–362.
- Lai YL, Yang SN, Liang JA, et al. Impact of body-mass factors on setup displacement in patients with head and neck cancer treated with radiotherapy using daily on-line image guidance. Radiat Oncol. 2014;9:19.
- Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136.
- Bourhis J, Sire C, Graff P, et al. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): an open-label phase 3 randomised trial. Lancet Oncol. 2012;13:145–153.
- Kataria T, Gupta D, Bisht SS, et al. Chemoradiation in elderly patients with head and neck cancers: a single institution experience. Am J Otolaryngol. 2015;36:117–121.
- Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25:4873–4879.
- Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66:981–991.
- Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J Clin Oncol. 2010;28:2732–2738.
- Chajon E, Lafond C, Louvel G, et al. Salivary gland-sparing other than parotid-sparing in definitive head-and-neck intensity-modulated radiotherapy does not seem to jeopardize local control. Radiat Oncol. 2013;8:132.
- Bjordal K, de Graeff A, Fayers PM, et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. EORTC Quality of Life Group. Eur J Cancer (Oxford, England: 1990). 2000;36:1796–1807.
- Castelli J, Simon A, Rigaud B, et al. A Nomogram to predict parotid gland overdose in head and neck IMRT. Radiat Oncol. 2016;11:79.
- Deasy JO, Moiseenko V, Marks L, et al. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76:S58–S63.
- Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl. 1985;8:S13–S19.
- Dijkema T, Raaijmakers CP, Ten Haken RK, et al. Parotid gland function after radiotherapy: the combined michigan and utrecht experience. Int J Radiat Oncol Biol Phys. 2010;78:449–453.
- Fitzpatrick JM, Hill DL, Shyr Y, et al. Visual assessment of the accuracy of retrospective registration of MR and CT images of the brain. IEEE Trans Med Imaging. 1998;17:571–585.
- Dice LR. Measures of the amount of ecologic association between species. Ecology.1945;26:297–302.
- Graves YJ, Smith AA, McIlvena D, et al. A deformable head and neck phantom with in-vivo dosimetry for adaptive radiotherapy quality assurance. Med Phys. 2015;42:1490–1497.
- Kirby N, Chuang C, Pouliot J. A two-dimensional deformable phantom for quantitatively verifying deformation algorithms. Med Phys. 2011;38:4583–4586.
- Tsuji SY, Hwang A, Weinberg V, et al. Dosimetric evaluation of automatic segmentation for adaptive IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2010;77:707–714.
- Lim JY, Leech M. Use of auto-segmentation in the delineation of target volumes and organs at risk in head and neck. Acta Oncol. 2016;55:799–806.
- Brock KK, Mutic S, McNutt TR, et al. Use of image registration and fusion algorithms and techniques in radiotherapy: Report of the AAPM Radiation Therapy Committee Task Group No. 132. Med Phys. 2017;44:e43–e76.
- Hvid CA, Elstrøm UV, Jensen K, et al. Cone-beam computed tomography (CBCT) for adaptive image guided head and neck radiation therapy. Acta Oncol. 2018;57(4):552–556.
- Brown E, Owen R, Harden F, et al. Predicting the need for adaptive radiotherapy in head and neck cancer. Radiother Oncol. 2015;116:57–63.
- Surucu M, Shah KK, Mescioglu I, et al. Decision trees predicting tumor shrinkage for head and neck cancer: implications for adaptive radiotherapy. Technol Cancer Res Treat. 2016;15:139–145.
- Pugachev A, Ruan S, Carlin S, et al. Dependence of FDG uptake on tumor microenvironment. Int J Radiat Oncol Biol Phys. 2005;62:545–553.
- Due AK, Vogelius IR, Aznar MC, et al. Recurrences after intensity modulated radiotherapy for head and neck squamous cell carcinoma more likely to originate from regions with high baseline [18F]-FDG uptake. Radiother Oncol. 2014;111:360–365.
- Madani I, Duthoy W, Derie C, et al. Positron emission tomography-guided, focal-dose escalation using intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:126–135.
- Vanderstraeten B, De Gersem W, Duthoy W, et al. Implementation of biologically conformal radiation therapy (BCRT) in an algorithmic segmentation-based inverse planning approach. Phys Med Biol. 2006;51:N277–N286.
- Geets X, Tomsej M, Lee JA, et al. Adaptive biological image-guided IMRT with anatomic and functional imaging in pharyngo-laryngeal tumors: impact on target volume delineation and dose distribution using helical tomotherapy. Radiother Oncol. 2007;85:105–115.
- Differding S, Sterpin E, Janssens G, et al. Methodology for adaptive and robust FDG-PET escalated dose painting by numbers in head and neck tumors. Acta Oncol. 2016;55:217–225.
- Duprez F, De Neve W, De Gersem W, et al. Adaptive dose painting by numbers for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;80:1045–1055.
- Rosen BS, Hawkins PG, Polan DF, et al. Early changes in serial CBCT-measured parotid gland biomarkers predict chronic xerostomia after head and neck radiotherapy. Int J Radiat Oncol Biol Phys. 2018;9. pii: S0360-3016(18)31049-6. doi: 10.1016/j.ijrobp.2018.06.048.
- Gros SA, Xu W, Roeske JC, et al. A novel surrogate to identify anatomical changes during radiotherapy of head and neck cancer patients. Med Phys. 2017;44:924–934.
- Piron O, Varfalvy N, Archambault L. Establishing action threshold for change in patient anatomy using EPID gamma analysis and PTV coverage for head and neck radiotherapy treatment. Med Phys. 2018;13. doi:10.1002/mp.13045 [epub ahead of print].
- Raaymakers BW, Jurgenliemk-Schulz IM, Bol GH, et al. First patients treated with a 1.5 T MRI-Linac: clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys Med Biol. 2017;62:L41–L50.
- Lagendijk JJ, Raaymakers BW, van Vulpen M. The magnetic resonance imaging-linac system. Semin Radiat Oncol. 2014;24:207–209.
- Lagendijk JJ, van Vulpen M, Raaymakers BW. The development of the MRI linac system for online MRI-guided radiotherapy: a clinical update. J Intern Med. 2016;280:203–208.
- Kupelian P, Sonke JJ. Magnetic resonance-guided adaptive radiotherapy: a solution to the future. Semin Radiat Oncol. 2014;24:227–232.