Abstract
Background: Kidney cancer rates in Estonia are high. The study aimed to examine long-term trends in kidney cancer incidence, mortality and survival in Estonia, with special focus on age, birth cohorts, morphology and TNM stage.
Material and methods: Estonian Cancer Registry provided data on all incident cases of kidney cancer (ICD-10 C64), diagnosed in adults (age ≥15 years) in Estonia during 1995 − 2014. Relative survival ratios (RSR) were calculated and excess hazard ratios of dying were estimated with gender, age, period of diagnosis and TNM stage as independent variables. Joinpoint regression modeling was used to calculate estimated annual percentage change for incidence (1970–2014) and mortality (1995–2016) trends. Age-specific incidence rates were presented by birth cohort and period of diagnosis.
Results: Incidence increased significantly in both sexes, with the steepest rise seen for localized cancer. Cohort effects were pronounced particularly in men, while period effects were seen from the mid-1980s to mid-1990s in both sexes. Age-standardized five-year RSR for total kidney cancer increased by 13 percentage units (from 53% to 65%) over the study period; the increase was larger for renal cell carcinoma (from 63% to 78%). Survival increases of about five percentage units were seen for stages I/II and III. Age and gender were not associated with excess risk of dying from renal cell carcinoma after adjusting for stage.
Conclusion: Estonia is currently among countries with the highest incidence of kidney cancer. The results suggest a combined effect of changing risk profiles in successive birth cohorts and increasing diagnostic activity around 1990. Large survival increase can mostly be attributed to earlier detection, but improved diagnosis and treatment have probably influenced stage-specific survival. High proportion of tumors with unspecified morphology and those with unknown stage among the elderly warrants further investigation of diagnostic and treatment practices.
Introduction
Globally, the total number of kidney cancer cases increased more than two-fold between 1990 and 2013, with comparable contributions from population growth, changes in population age structure and increasing incidence rates [Citation1]. Increased use of imaging techniques has contributed to the rising incidence of kidney cancer in developed countries, where most of the increase can be attributed to localized tumors [Citation2], and up to half of the new cases are detected as incidental diagnoses [Citation3]. However, this phenomenon cannot explain the incidence rises observed in low- or medium-resource settings [Citation1,Citation4], where the growing prevalence of risk factors is a more likely explanation [Citation5].
The majority (90%) of kidney cancers are renal cell carcinomas (RCC) [Citation6]. Established risk factors for RCC include tobacco smoking, obesity and hypertension [Citation5]. Smoking increases the risk of RCC by 38% [Citation7]. A five-kg/m2 increase in body mass index (BMI) was shown to increase kidney cancer risk by 24% in men and 34% in women [Citation8]. Elevated blood pressure was associated with a two-to-three-fold risk of RCC independently from other risk factors [Citation9]. A small proportion of RCC arises in populations with familial cancer syndromes, such as von Hippel–Lindau, and birth defects like having a horseshoe kidney [Citation10]. Genetic susceptibility and its interaction with other risk factors may also have a role in non-familial RCC etiology [Citation5]. In addition, the influence of factors such as occupational carcinogens, medication use (e.g., non-steroidal anti-inflammatory drugs) and dialysis has been suggested [Citation5,Citation11].
Kidney cancer accounts for 4% of total new cancer cases in Estonia today [Citation12]. The incidence rates are high, particularly in men: according to Cancer in Five Continents Volume XI, kidney cancer incidence in Estonia ranked fifth in Europe among men and eighth among women [Citation13]. Kidney cancer mortality in Estonia is also among the highest in Europe [Citation14].
According to EUROCARE-3, the five-year relative survival for patients diagnosed in 1990–1994 was just over 40% in Estonia, while the European average was 57% [Citation15]. In EUROCARE-5, however, the Estonian estimate was already equal to the European average (61%) [Citation16].
The objective of this study was to examine long-term trends in kidney cancer incidence, mortality and survival in Estonia, with special focus on age, birth cohorts, morphology and TNM stage.
Material and methods
Data on all incident cases of kidney cancer (ICD-10 code C64), diagnosed in adults (age ≥15 years) in Estonia during 1995 − 2014, were obtained from the Estonian Cancer Registry (ECR), which is population-based, covers the whole country (population 1.34 million according to the 2011 census) and has data since 1968. Reporting to the registry is obligatory for all physicians and pathologists in Estonia. Multiple sources are used for case ascertainment, including linkages with the electronic patient records of two cancer centers and trace-back of cases first identified via death certificates. The registry uses ICD-O-3 for coding topography and morphology and follows international definitions and rules, issued by the International Association of Cancer Registries and the European Network of Cancer Registries [Citation17], for reporting incidence and survival.
Percentage of microscopically verified cases (%MV), percentage of cases based on death certificate only (%DCO) and percentage of cases diagnosed at autopsy were used to estimate data quality. For descriptive purposes, age was categorized into six groups (15–44, 45–54, 55–64, 65–74, 75–84, ≥85 years); the two youngest and two oldest groups were collapsed for further analyses. Morphology was grouped into renal cell carcinoma (RCC; ICD-O-3 morphology codes 8260/3, 8290/3, 8310/3, 8312/3, 8317/3, 8319/3) [Citation6]; not otherwise specified (NOS; 8000/3–8005/3); and other specified (all other morphologies). Stage was coded based on the 7th version of the TNM classification [Citation18]: stage I (T1N0M0); stage II (T2N0M0); stage III (T3N0M0; T1-3N1M0); stage IV (T4NanyM0; TanyN2M0; TanyNanyM1). Due to the change in the definition of T1 tumors between the 4th and 5th version of the TNM classification (published in 1992 and 1997, respectively) [Citation19,Citation20], stages I and II were combined in trend analyses. Stages I and II are shown separately for 2010–2014.
‘Surgery done’ includes all cases with reported surgical treatment, regardless of intention. Due to the passive nature of reporting, it was not possible to distinguish between cases with ‘surgery not done’ and ‘unknown if surgery done’.
Follow-up for vital status from the date of diagnosis through 2014 was conducted by the ECR at the Estonian Population Registry using unique personal identification numbers. In case of death or emigration, the respective dates were obtained.
Relative survival ratios (RSR) were calculated as the ratio of the observed survival of cancer patients to the expected survival of the underlying population. The latter estimate was calculated according to the Ederer II method using national life tables stratified by single year of age and calendar year [Citation21]. RSRs with 95% confidence intervals (CI) were estimated using cohort analysis for patients diagnosed during 1995 − 1999, 2000 − 2004 and 2005 − 2009, and period analysis for 2010 − 2014 [Citation22]. DCO and autopsy cases were excluded from survival analyses. Patients who were diagnosed and died on the same day were included with one day of survival time. Chi-square test was used to test the significance of the difference between distributions and two-sided p-values to compare proportions.
Excess hazard ratios (EHR) of dying within five years after diagnosis were estimated in the framework of generalized linear models using a Poisson assumption for the number of observed deaths [Citation23]. Cases with unknown stage were excluded. Gender, age, period of diagnosis and TNM stage were included as independent variables. All models included year of follow-up. The EHRs are presented with 95% CI for all kidney cancers and for RCC. Interactions between variables were tested with likelihood ratio test.
STATA 14.2 (StataCorp, College Station, Texas, USA) was used for all analyses; survival analysis was done using the strs algorithm [23]. International Cancer Survival Standards were used for age-standardization [Citation24].
Age-specific and age-standardized (world) incidence (1970–2014) and mortality (1995–2016) rates were modeled, and the estimated annual percentage change (APC) and average annual percentage change (AAPC) with 95% confidence intervals (CI) calculated with Joinpoint Regression Program (version 4.1.1.1) from the Surveillance Research Program of the US National Cancer Institute (http://surveillance.cancer.gov/joinpoint/). The analysis of time trends involved presentation of age-specific incidence rates by period of diagnosis and birth cohort, which enabled graphical exploration of the age, period and cohort effects.
Data on kidney cancer deaths were obtained from the Causes of Death Registry, and population denominator data from Statistics Estonia.
The study protocol was approved by the Tallinn Medical Research Ethics Committee.
Results
The total number of kidney cancer cases diagnosed in adults in Estonia during 1995–2014 was 5326 (). The %MV and %Autopsy increased significantly over the study period, while %DCO remained stable. Gender distribution did not change, but there was a shift towards older age at diagnosis, as the proportion of cases diagnosed at age ≥75 years increased significantly. Median age at diagnosis increased from 65 to 69 years. The proportion of RCC increased, mostly on the account of the NOS subtype. Stage distribution shifted significantly towards earlier stages, while the proportion of unknown stage did not change. The proportion of patients with known surgery increased.
Table 1. Incident cases of kidney cancer in adults (age ≥15 years) in Estonia, 1995–2014.
Overall, female kidney cancer patients were significantly older than male patients (Supplementary Table 1). Median age at diagnosis increased from 64 to 66 years in men and from 67 to 73 years in women. Compared to men, women had higher proportion of NOS tumors and were diagnosed at earlier stages than men.
The %MV ranged from 92% in patients age 15–54 to 63% in age group ≥75 (p < .001). Morphology distribution differed significantly across age groups as the proportion of NOS tumors was 9% in the youngest and 39% in the oldest age group. Significant differences between age groups were also observed in stage distribution: the proportion of cases diagnosed at stages I/II ranged from 53% in patients aged 15–54 to 38% in age group ≥75 (p < .001); the proportion of cases with unknown stage was 8% and 22%, respectively (p < .001). The proportion of patients reported to have surgery was 88%, 82%, 77% and 54% in the four age groups, respectively (p < .001).
In 2014, the age-standardized incidence was 18/100,000 in men and 9/100,000 in women; the respective mortality rates in 2016 were 6/100,000 and 2/100,000. Incidence increased significantly in men from 1970 to 2014, with the steepest rise during 1986–1996 (APC 6.0) (). In women, incidence increased significantly until 1996, declined somewhat until 2001, and increased significantly thereafter (APC 2.2). Mortality decreased significantly in men throughout the study period, but female rates have plateaued since 2002. shows cohort effects with age-specific incidence rates increasing in successive birth cohorts, particularly in men, and period effects from the mid-1980s to mid-1990s in most age groups.
Figure 1. (A) Observed (dashed line) and modeled (solid line) age-standardized (World) rates and annual percentage change (APC) with 95% confidence intervals for kidney cancer incidence (1970–2014) and mortality (1995–2014) trends in Estonia; (B) Age-specific kidney cancer incidence trends by five-year birth cohort and period of diagnosis in Estonia, 1970–2014; (C) Observed (dashed line) and modeled (solid line) age-standardized (World) rates and annual percentage change (APC) with 95% confidence intervals for stage-specific kidney cancer incidence trends in Estonia, 1970–2014. *The APC is significantly different from zero at alpha =0.05.
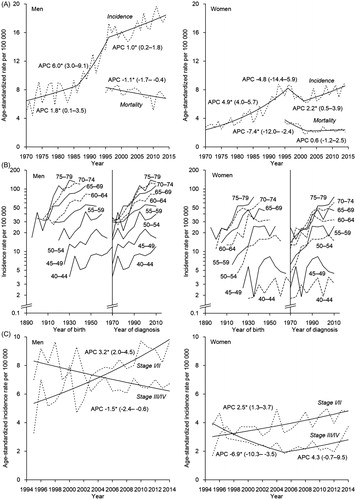
The age-standardized incidence increased rapidly for stage I/II cancer in both sexes from 1995 to 2014; there was a steady downward trend for more advanced stages in men, but not in women (). Age-specific mortality decreased in all men between ages 15 and 74 years from 1995–2014. In the elderly, the rates fluctuated but resulted in an overall upward trend (AAPC 2.9). Among women, mortality decreased in all women younger than 74 years, while in age group ≥75, the downward trend turned to a significant rise from 2007 (APC 8.7).
Age-standardized five-year RSR increased by 13 percentage units for total kidney cancer and 15% units for RCC (). Women had higher five-year RSRs than men throughout the study period and their survival gain was also larger, particularly for RCC. The latest five-year RSRs for the youngest and oldest age groups differed by 28 percentage units for all kidney cancer, but only by 5% units for RCC. Modest increases were seen for stages I/II and III. In 2010–2014, the difference between the five-year RSR of all patients and surgically treated patients was 16 percentage units for all kidney cancer and four percentage units for RCC, while the survival gain for surgically treated patients was of the same magnitude as for all patients.
Table 2. Five-year relative survival ratios (RSR) for all kidney cancers and renal cell carcinoma in Estonia, 1995–2014.
In 2010–2014, 42% of all kidney cancers and 51% of RCC were diagnosed at stage I (9% and 10% at stage II, respectively). The five-year RSRs for stages I and II were 94% and 85% for all kidney cancers, and 95% and 87% for RCC.
Modeling excess mortality for all kidney cancers indicated that the significant survival advantage of women in un-adjusted analyses was explained by differences in the distribution of other factors (). In the adjusted model for all kidney cancers, the EHR of dying was significantly associated with age, stage and calendar period. The respective model for RCC showed a significant association only with calendar period and stage.
Table 3. Excess hazard ratio (EHR) of death within five years of diagnosis among patients with kidney cancer in Estonia, 1995–2014.
Discussion
In the mid-1980s, Estonia was among countries with average male kidney cancer incidence in Europe, but the rate had nearly doubled and risen to one of the highest in the continent by 2008–2012 [Citation13]. Our long-term trend analyses revealed that although the steepest rise in incidence occurred from the mid-1980s to mid-1990s, the increase continued into the most recent years. The 2014 age-standardized incidence rate for kidney cancer in men in Estonia (18/100,000) was considerably higher than in neighboring Finland (10/100,000) [Citation25]. Most developed countries have seen increases in kidney cancer incidence over the past decades [Citation4]. The observed birth-cohort-specific and period-specific effects suggest a combined effect of individual risk factors and increased incidental detection of indolent tumors [Citation26]. Our findings support previous observations. The recent stabilization of incidence trends in several high-incidence countries suggests a more conservative use of imaging and mitigation of over-diagnosis [Citation26]. High autopsy detection rate indicates the prevalence of slow-growing tumors in the population [Citation27]. Despite significant increase over the study period, the proportion of cases detected at autopsy remained below 2% in our data, which is similar to Norway [Citation25]. Overall autopsy rate in Estonia has decreased from 34% to 18% between 1989 and 2015; the latter estimate is close to that of Finland (21%), Czech Republic (17%) and European Union as a whole (15%) [Citation28].
Ultrasonography became widely used in Estonia in early 1990s. The proportion of kidney cancer cases primarily diagnosed by abdominal ultrasonography in regional hospitals increased from 21% to 82% from late 1980s to late 1990s [Citation29]. The use of abdominal computed tomography (CT) as a primary diagnostic method increased over the same period from zero to 11% [Citation29], suggesting a very limited availability of CT even in late 1990s. Currently, the availability of CT scanners and MRI units in Estonia is below the OECD average, but the total number of CT exams per 1000 population was slightly higher than the OECD average both in 2009 and in 2015 [Citation30].
The relatively low microscopic verification rate in our data raises doubt whether some of the clinically diagnosed tumors might not have been malignant. Up to 30% of surgically treated small (<4 cm) tumors were reported to be benign in the Nordic countries [Citation31]. However, the low survival of NOS tumors in Estonia contradicts this suggestion.
Tobacco smoking has shown a strong dose-dependent effect on kidney cancer risk, which is much higher in men than in women [Citation7]. The proportion of daily smokers among adult men in Estonia decreased from 46% in 1990–1994 to 30% in 2016, whereas in women the change was much smaller (from 18% to 16%) [Citation32]. Nevertheless, the role of smoking in recent kidney cancer trends is debatable as lung cancer trends contrast with kidney cancer trends [Citation33].
Obesity is another established risk factor that is linked to kidney cancer in a dose-dependent manner [Citation8]. In France, over 20% of all kidney cancer cases could be attributed to high BMI [Citation34]. It is likely that the increasing burden of obesity in Estonia has influenced recent trends. The prevalence of self-reported obesity (BMI ≥30) in men age 16–64 in Estonia increased from 11% in 1990–1994 to 20% in 2016; in women, the respective increase was less pronounced (from 17% to 19%) [Citation32]. High prevalence of obesity and central obesity was also found in a cross-sectional study using direct measurements [Citation35].
Hypertension affects kidney cancer risk independently from obesity [Citation5], whereas there have been indications that adequate blood pressure control lowers the risk [Citation9]. Mortality from hypertension and other cardiovascular diseases in Estonia remains high, despite declining trends [Citation30]. A cross-sectional study reported high prevalence of hypertension among men and women aged 20–65 in Estonia (44% and 29%, respectively) [Citation36]. In Czech Republic where kidney cancer rates have been the highest in Europe [Citation4], a consecutive series of cross-sectional surveys indicated high prevalence of hypertension both in males and females, despite a significant decline observed from 1980s to early 2000s [Citation37].
In many high-income countries, kidney cancer mortality has been decreasing during past decades [Citation4,Citation38]. We observed a steady decline in mortality among men, despite the rising trend in the ≥75 age group. Among women, however, mortality decline has been replaced by a plateau since 2002, owing to the rapid rise in age group ≥75. This type of age-specific trends has not been evident in any of the Nordic countries [Citation25] or in the Czech Republic but was also seen in Latvia [Citation14].
We observed the fastest growing incidence rates for localized kidney cancer, whereas the trends for more advanced tumors varied. In the US, incidence increase was limited to localized tumors [Citation2]. The proportion of stage I/II kidney cancers increased rapidly early in our study period and gradually thereafter, reaching 52% by the most recent period (60% among cases with known stage, data not shown). The latter estimate is slightly higher than in the UK (56%) [Citation39] and just below the Czech estimate for 2005–2008 (63%) [Citation40]. The proportion of stage I/II among RCC cases was 61% (data not shown), compared to 70% in the US in 2006 [Citation2]. These indices do not suggest overly intensive detection of indolent tumors in Estonia as an explanation for the observed high incidence.
The most recent five-year RSR estimates for kidney cancer in Estonia are similar to those observed in Finland [Citation25] and higher than in the UK [Citation39]. Survival was very low (five-year RSR below 30%) in Estonia throughout the 1970s and 1980s [Citation41]; in EUROCARE-2, the Estonian estimates were the lowest in Europe [Citation42]. This was at least partly due to the very high proportion of stage IV cancers: in 1986–1988, the proportion of primary kidney cancers diagnosed at stage IV at Estonian hospitals was close to 60% [Citation29]. Another possible contributing factor was the low availability of accurate diagnostic methods, causing inadequate staging and subsequent non-optimal treatment. However, substantial improvements in survival were observed since the end of 1980s [Citation41], which continued from 1995–1999 to 2000–2004 according to our results, particularly for RCC. This early survival increase was likely mainly driven by the shift towards earlier stages, as also indicated by the multivariate model. Improvement in stage-specific survival was more modest compared to overall estimates, which also suggests the role of stage shift, and possible lead-time and length bias. The stage-specific RSRs for all kidney cancers shown for the Czech Republic in 2005–2008 were close to our estimates for 2005–2009 [Citation40]. Nephrectomy has remained central in the management of kidney cancers, with increasing application of partial nephrectomy for early tumors to maintain organ function [Citation43]. A recent survey in the Nordic countries found that 40% of all tumor excisions were nephron-sparing surgeries [Citation31]. In late 1990s, radical and simple nephrectomies accounted for over 95% of all kidney cancer surgeries in Estonia [Citation29]; no data are available for more recent periods.
The introduction of modern targeted therapies has greatly improved the prognosis of patients with metastatic RCC. In Estonia, interferon alpha-2A (INFa2A) monotherapy [Citation44], was the standard reimbursed medical treatment until 2008. As of 2008, sorafenib [Citation45] has been added to the second-line treatment of metastatic RCC. From late 2009, INFa2A and bevacizumab combination [Citation46] and sunitinib [Citation47] monotherapy have been employed as the first-line treatment in patients with low- and intermediate-risk, and temsirolimus [Citation48] in patients with high-risk metastatic RCC. Pazopanib [Citation49] treatment became routinely available from late 2012 and axitinib [Citation50] from 2014. Thus, the implementation of new generations of medical therapies has been relatively fast in Estonia.
Gender survival gap was larger in our data (six percentage units in 2010–2014) than observed in EUROCARE-5 (two percentage units) [Citation16] or in Canada (three percentage units) [Citation38]. Men had less favorable stage distribution and female survival advantage was no longer evident after adjusting for age, stage and calendar period in the multivariate model.
Our findings also demonstrated a wide age gap in survival, similar to EUROCARE-5 [Citation16] and the Canadian study [Citation38]. This was expected, given the significant association of age with %MV, stage and morphology distribution as well as known surgery. Variation across age groups was much smaller for RCC than for total kidney cancer, and even the oldest age group experienced large survival gain for RCC. Excess mortality did not vary across age groups among RCC patients in multivariate analysis. These findings suggest favorable outcomes for elderly patients whose general health condition is good enough to undergo treatment. It is likely that rigorous diagnostic procedures and standard treatments are not applied to many patients in the older age group. This may be due to comorbidities and poor performance status, which can be an important barrier to cancer-specific treatment [Citation51]. There are no data available on the frequency of pretreatment biopsies in Estonia. According to the 2017 guidelines of the European Urological Association [Citation43], renal biopsy is not indicated for comorbid and frail patients who can be considered only for conservative management regardless of biopsy results. Nonetheless, in Norway, the proportion of patients with no histopathological verification of metastatic disease is decreasing among the elderly [Citation52].
The main strength of the study was the use of population-based nationwide cancer registry data collected uniformly over the study period. The large proportion of NOS tumors and low %MV among the elderly likely reflects clinical practices rather than the quality of CR data, as the registry collects information from multiple resources, including pathological laboratories. Another strength of the study was the ability to examine incidence and survival by TNM stage. The study was limited by the absence of information on whether cancer detection was symptomatic or incidental, more detailed treatment information, tumor diameter and grade. The estimates for surgically treated patients should be interpreted with caution as surgery data may have been underreported. As with other register-based studies, we had no individual information on risk factors or comorbidities. In birth cohort analyses, incidence rates of earlier and most recent cohorts are less reliable due to fewer age groups.
In conclusion, Estonia is currently among countries with the highest incidence of kidney cancer. The long-term upward trends in incidence observed in both genders suggest a combined effect of changing risk profiles in successive birth cohorts and higher diagnostic activity during certain periods, particularly around 1990, when modern imaging techniques became widely available in Estonia. Large survival increase seen over past decades can mostly be attributed to earlier detection, but improved diagnosis and treatment have probably influenced stage-specific survival. Despite general progress in kidney cancer care, overall mortality remains high and survival remains low among elderly patients. It warrants further investigation whether the low diagnostic accuracy and treatment activity among the elderly is due to frailty and comorbid conditions of the patients or some other factors.
Supplemental Material
Download MS Word (61.7 KB)Acknowledgments
The authors thank Dr. Margit Mägi and Mrs. Pille Härmaorg from the Estonian Cancer Registry for providing the data.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Dy GW, Gore JL, Forouzanfar MH, et al. Global burden of urologic cancers, 1990–2013. Eur Urol. 2017;71:437–446.
- Sun M, Thuret R, Abdollah F, et al. Age-adjusted incidence, mortality, and survival rates of stage-specific renal cell carcinoma in North America: a trend analysis. Eur Urol. 2011;59:135–141.
- Rabjerg M, Mikkelsen MN, Walter S, et al. Incidental renal neoplasms: is there a need for routine screening? A Danish single-center epidemiological study. Apmis. 2014;122:708–714.
- Znaor A, Lortet-Tieulent J, Laversanne M, et al. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67:519–530.
- Chow W, Dong L, Devesa S. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245–257.
- Eble JN, Sauter G, Epstein JI, et al. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004.
- Hunt JD, van der Hel OL, McMillan GP, et al. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer. 2005;114:101–108.
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578.
- Weikert S, Boeing H, Pischon T, et al. Blood pressure and risk of renal cell carcinoma in the European prospective investigation into cancer and nutrition. Am J Epidemiol. 2008;167:438–446.
- Cho E, Lindblad P, Adami H-O. Kidney cancer. In: Adami H-O, Hunter P, Trichopoulos D, eds. Textbook of Cancer Epidemiology 2. Oxford: Oxford University Press; 2008. p. 597–612.
- Choueiri TK, Je Y, Cho E. Analgesic use and the risk of kidney cancer: a meta-analysis of epidemiologic studies. Int J Cancer. 2014;134:384–396.
- National Institute for Health Development. Health statistics and health research database [Internet]. Tallinn: National Institute for Health Development; 2018 [cited 2018 May 14]. Available from: http://pxweb.tai.ee/PXWeb2015/index_en.html
- Bray F, Colombet M, Mery L, et al. Cancer Incidence in Five Continents, Vol.XI (electronic version). Lyon: International Agency for Research on Cancer, 2017.
- International Agency for Research on Cancer. World Health Organization Mortality database (accessed on 2016 Sep 9) [Internet]. Lyon: International Agency for Research on Cancer; 2018 [cited 2018 May 14]. Available from: http://www-dep.iarc.fr/WHOdb/WHOdb.htm
- Sant M, Aareleid T, Berrino F, et al. EUROCARE-3: survival of cancer patients diagnosed 1990–94 - results and commentary. Ann Oncol. 2003;14:61v–118.
- Marcos-Gragera R, Mallone S, Kiemeney LA, et al. Urinary tract cancer survival in Europe 1999–2007: results of the population-based study EUROCARE-5. Eur J Cancer. 2015;51:2217–2230.
- International Agency for Research on Cancer, World Health Organization, International Association of Cancer Registries, European Network of Cancer Registries. International rules for multiple primary cancers (ICD-O Third Edition). Lyon: International Agency for Research on Cancer; 2004.
- International Union Against Cancer (UICC). TNM Classification of Malignant Tumours, 7th edition. New York: Wiley Blackwell; 2009.
- American Joint Committee on Cancer. Manual for staging of cancer, Fourth Edition. Philadelphia: American Joint Committee on Cancer; 1992.
- American Joint Committee on Cancer. AJCC Cancer Staging Manual, Fifth Edition. Philadelphia: American Joint Committee on Cancer; 1997.
- Ederer F, Heise H. Instructions to IBM 650 programmers in processing survival computations. Methodological note no. 10. Bethesda (MD): End Results Evaluation Section, National Cancer Institute; 1959.
- Brenner H, Gefeller O, Hakulinen T. Period analysis for “up-to-date” cancer survival data: theory, empirical evaluation, computational realisation and applications. Eur J Cancer. 2004;40:326–335.
- Dickman PW, Coviello E. Estimating and modeling relative survival. Stata J. 2015;15:186–215.
- Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer. 2004;40:2307–2316.
- Engholm G, Ferlay J, Christensen N, et al. NORDCAN: cancer incidence, mortality, prevalence and survival in the nordic countries, version 7.3 (2016 Aug 7) [Internet]. Association of the Nordic cancer registries. Cope hagen: Danish Cancer Society; 2018 [cited 2018 April 20]. Available from: http://www.ancr.nu
- Znaor A, Laversanne M, Bray F. Less overdiagnosis of kidney cancer? An age-period-cohort analysis of incidence trends in 16 populations worldwide. Int J Cancer. 2017;141:925–932.
- Engholm G, Hakulinen T, Gislum M, et al. Trends in the survival of patients diagnosed with kidney or urinary bladder cancer in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol. 2010;49:655–664.
- Autopsy rate (%) for all deaths. European Health Information Gateway [Internet]. WHO Regional Office for Europe, Division of Information, Evidence, Research and Innovation; 2018 [cited 2018 April 20]. Available from: https://gateway.euro.who.int/en/indicators/hfa_545-6410-autopsy-rate-for-all-deaths/
- Padrik P, Kirsimägi Ü, Everaus H. Changes in the natural history of renal cancer: comparison of Estonian data from the periods 1986–1988 and 1996–1998. Int Urol Nephrol. 2007;39:35–41.
- OECD. Health at a Glance 2017. OECD Indicators. Paris: OECD Publishing; 2018.
- Nisen H, Järvinen P, Fovaeus M, et al. Contemporary treatment of renal tumors: a questionnaire survey in the Nordic countries (the NORENCA-I study). Scand J Urol. 2017;51:360–366.
- Tekkel M, Veideman T. Health Behavior among Estonian Adult Population, 2016. Tallinn: National Institute for Health Development; 2017.
- Aareleid T, Zimmermann M-L, Baburin A, et al. Divergent trends in lung cancer incidence by gender, age and histological type in Estonia: a nationwide population-based study. BMC Cancer. 2017;17:596.
- Arnold M, Touillaud M, Dossus L, et al. Cancers in France in 2015 attributable to high body mass index. Cancer Epidemiol. 2018;52:15–19.
- Kaldmäe M, Zemtsovskaja G, Abina J, et al. Prevalence of cardiovascular disease risk factors in Tallinn, Estonia. Medicina (Kaunas). 2017;53:268–276.
- Kaldmäe M, Annuk M, Viigimaa M, et al. Prevalence and determinants of hypertension in Estonian adults. Scand J Public Health. 2014;42:504–510.
- Cífková R, Skodová Z, Lánská V, et al. Trends in blood pressure levels, prevalence, awareness, treatment, and control of hypertension in the Czech population from 1985 to 2000/01. J Hypertens. 2004;22:1479–1485.
- De P, Otterstatter MC, Semenciw R, et al. Trends in incidence, mortality, and survival for kidney cancer in Canada, 1986–2007. Cancer Causes Control. 2014;25:1271–1281.
- Kidney cancer statistics [Internet]. Cancer Research UK; 2018 [cited 2018 April 5]. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/kidney-cancer
- Pavlík T, Májek O, Büchler T, et al. Trends in stage-specific population-based survival of cancer patients in the Czech Republic in the period 2000–2008. Cancer Epidemiol. 2014;38:28–34.
- Aareleid T, Brenner H. Trends in cancer patient survival in Estonia before and after the transition from a Soviet republic to an open-market economy. Int J Cancer. 2002;102:45–50.
- Berrino F, Capocaccia R, Esteve J, et al. Survival of cancer patients in Europe: the EUROCARE-2 study. IARC Scientific Publications No. 151. Lyon: International Agency for Research on Cancer; 1999.
- Oncology Guidelines. Renal Cell Carcinoma. 2017. European Association of Urology; [cited 2018 April 20]. Available from: http://uroweb.org/guideline/renal-cell-carcinoma/
- Motzer BRJ, Bacik J, Murphy BA, et al. Interferon-Alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. Jco. 2002;20:289–296.
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134.
- Escudier B, Bellmunt J, Négrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. Jco. 2010;28:2144–2150.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. Jco. 2009;27:3584–3590.
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. Jco. 2010;28:1061–1068.
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939.
- Audisio RA, Bozzetti F, Gennari R, et al. The surgical management of elderly cancer patients: recommendations of the SIOG surgical task force. Eur J Cancer. 2004;40:926–938.
- Beisland C, Johannesen TB, Reisaeter LAR, et al. Real-life use of diagnostic biopsies before treatment of kidney cancer: results from a Norwegian population-based study. Scand J Urol. 2018;52:38–44.
