The presentation of a bone sarcoma during pregnancy is very rare and a challenging situation for all parties involved. Here, we describe a case of a patient who was diagnosed with bone sarcoma during pregnancy and received a tailored multidisciplinary treatment.
A 31-year-old previously healthy Caucasian woman, G2P1, was diagnosed with a high-grade pleomorphic undifferentiated spindle cell sarcoma of the distal femur at 22 weeks of gestation. No metastases were found on a whole-body fluorodeoxyglucose (FDG)-positron emission tomography (PET)-scan combined with low-dose computerized tomography (CT)-scan. Given the clinicopathological characteristics of the tumor, an osteosarcoma protocol was applied. A multidisciplinary team was formed, consisting of a medical oncologist, gynecologist–obstetrician, orthopedic oncologist, pharmacologist and specialized adolescent and young adults (AYA) nurse. After thorough counseling, the patient decided to maintain the pregnancy. The usual neoadjuvant schedule of chemotherapy (cisplatin, doxorubicin and methotrexate) [Citation1] was adjusted in close collaboration with the pharmacologist to minimize the risk of teratogenic effects. Cisplatin and doxorubicin can be used safely, though with caution, from the second trimester of pregnancy [Citation2,Citation3]. Since limited data are available on the administration of the high dose of 100 mg/m2 cisplatin during pregnancy, and to avoid high peak concentrations of cisplatin, the dosage of 100 mg/m2 was divided over five dosages of 20 mg/m2 administered over five consecutive days (day 1–5 q 21 d). In order to determine the systemic exposure to free fraction platinum (Pt) in this patient, venous blood samples were obtained during cisplatin infusion. The calculated free platinum area under the concentration time curve (AUC0-24h) after a single dose of cisplatin of 20 mg/m2 was 0.64 µg*h/ml. The extrapolated AUC0–120h of 100 mg/m2 given over five consecutive days was thereby 3.19 µg*h/ml. This is considered to be adequate exposure [Citation4]. Because of its highly teratogenic properties, high dose methotrexate was postponed until after delivery.
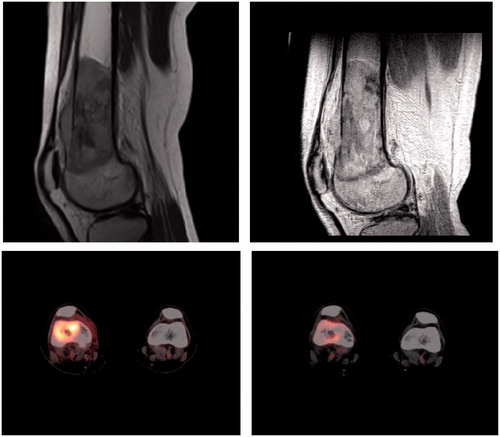
During chemotherapy, fetal well-being was monitored by the gynecologist. Two courses of chemotherapy resulted in a metabolic but not volumetric response of the tumor (). At the pregnancy term of 29 + 5 weeks, we proceeded to limb saving surgery with a mega-prosthesis reconstruction. Fetal monitoring during and after surgery did not show any negative effects for the baby. The diagnosis of a high-grade pleomorphic undifferentiated spindle cell sarcoma was confirmed by the pathologist, with resection margins of at least 2 cm and a therapy effect of 50–60% necrosis, the latter below the preferred percentage of >90%. Because of the necessity of highly teratogenic adjuvant chemotherapy after surgery, prematurely cesarean section was scheduled at 32 + 0 weeks of gestation and a healthy daughter was delivered (weight 1870 g, p50–80, Apgar score 8/9 after 1/5 min). Due to prematurity, but not dysmaturity, the baby was admitted to the Neonatal Care Unit for almost 7 weeks. No adverse effects, especially no hearing impairment, were discovered until the age of 2 years, whereafter she was discharged from further pediatric follow-up.
Figure 1. MRI and PET scan showing the tumor before treatment (left) and after two courses of chemotherapy (right).
Eleven days after delivery, adjuvant chemotherapy was started. The treatment protocol was adjusted to comply to the cumulative dosages used in the EURAMOS protocol, which was considered of extra importance since the necrosis percentage was <90% [Citation1]. Furthermore, intensive revalidation was started.
The total treatment plan took 10 months and was challenging for the patient, her family and the multidisciplinary team, and personalized supportive AYA care was offered to them to enable mother and child well-being. By now, we know how important this period has been, since 2 years after completion of treatment, pulmonary and bone metastases were detected for which the patient is currently treated with palliative intent.
Bone sarcomas are extremely rare during pregnancy with only 75 cases reported since 1963. Chemotherapeutic treatment during pregnancy has only been described in four cases of Ewing sarcomas () [Citation5–8], despite the fact that a multidisciplinary approach including chemotherapy is standard of care for localized bone sarcomas.
Chemotherapy in pregnancy is safe [Citation9], but needs empirical pharmacological adjustments and careful monitoring. The use of platinum-based chemotherapy during pregnancy is associated with an increased incidence of notification of small for gestational age (OR 3.12, 95% CI 1.45–6.70) [Citation10]. Concerning the long-term effects of exposure to maternal chemotherapy, no significant effects on children’s general health, growth, hearing, cardiac function or central nervous system were found [Citation9], thus indicating that chemotherapy can be safely administered after the first trimester.
However, prematurity was significantly associated with impaired cognitive development [Citation9]. The overall frequency of premature birth in patients with cancer during pregnancy is rather high, around 50% [Citation10]. As happened in our case, 88% of premature births were scheduled deliveries based on medical considerations for mother and child [Citation10]. These findings emphasize the importance of carefully considering the timing of delivery.
Despite our appropriate cisplatin AUC0–120h of 3.19 µg*h/ml for the divided doses cisplatin, the percentage of necrosis found in the pathology sample was below the required 90%. In comparison, 50% of the non-pregnant patients treated within the EURAMOS trial had less than 90% necrosis after neoadjuvant doxorubicin, cisplatin and high dose methotrexate. Our patient had the same cumulative methotrexate dose although administered only in the adjuvant setting. Less than 90% necrosis is associated with poor outcome [Citation11].
Surgical treatment of the tumor during pregnancy is safe, but needs consideration regarding timing with respect to both pregnancy term and fetal monitoring, as well as the expected nadir of the chemotherapy. If possible, surgery should be delayed until the second trimester to decrease the risk of teratogenicity and miscarriage due to anesthetics [Citation12]. Effects of the oncologic surgery on the way of delivery of the child in a later phase should also be taken in account, e.g., the newly mega-prosthesis of our patient inhibited vaginal delivery.
With this case we emphasize the importance of a patient-centered approach with an adjusted multidisciplinary team, in the unique setting of a pregnant patient with a curable but unfavorable high grade undifferentiated sarcoma of the bone.
Consent
The patient whose case is described in the case report has provided written informed consent for anonymous publication.
Table 1. Reported single cases of bone sarcoma during pregnancy.
Disclosure statement
No potential conflict of interests was reported by the authors.
References
- Whelan JS, Bielack SS, Marina N, et al. EURAMOS-1, an international randomised study for osteosarcoma: results from pre-randomisation treatment. Ann Oncol. 2015;26:407–414.
- Mir O, Berveiller P, Ropert S, et al. Use of platinum derivatives during pregnancy. Cancer. 2008;113:3069–3074.
- Germann N, Goffinet F, Goldwasser F. Anthracyclines during pregnancy: embryo-fetal outcome in 160 patients. Ann Oncol. 2004;15:146–150.
- Schellens JH, Ma J, Planting AS, et al. Relationship between the exposure to cisplatin, DNA-adduct formation in leucocytes and tumour response in patients with solid tumours. Br J Cancer. 1996;73:1569–1575.
- Schur S, Wild J, Amann G, et al. Sarcoma of the Ewing family in pregnancy: a case report of intrauterine fetal death after induction of chemotherapy. Case Rep Oncol. 2012;5:633–638.
- Nakajima W, Ishida A, Takahashi M, et al. Good outcome for infant of mother treated with chemotherapy for Ewing sarcoma at 25 to 30 weeks' gestation. J Pediatr Hematol Oncol. 2004;26:308–311.
- Merimsky O, Le Chevalier T, Missenard G, et al. Management of cancer in pregnancy: a case of Ewing's sarcoma of the pelvis in the third trimester. Ann Oncol. 1999;10:345–350.
- Haerr RW, Pratt AT. Multiagent chemotherapy for sarcoma diagnosed during pregnancy. Cancer. 1985;56:1028–1033.
- Amant F, Van Calsteren K, Halaska MJ, et al. Long-term cognitive and cardiac outcomes after prenatal exposure to chemotherapy in children aged 18 months or older: an observational study. Lancet Oncol. 2012;13:256–264.
- de Haan J, Verheecke M, Van Calsteren K, et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol. 2018;19:337–346.
- Whelan JS, Jinks RC, McTiernan A, et al. Survival from high-grade localised extremity osteosarcoma: combined results and prognostic factors from three European Osteosarcoma Intergroup randomised controlled trials. Ann Oncol. 2012;23:1607–1616.
- Cheek TG, Baird E. Anesthesia for nonobstetric surgery: maternal and fetal considerations. Clin Obstetr Gynecol. 2009;52:535–545.
- Lysyj A, Bergquist JR. Pregnancy complicated by sarcoma. Report of two cases. Obstet Gynecol. 1963;21:506–509.
