Abstract
Objectives: To examine patterns of care associated with the administration of proton versus photon therapy for adult patients with primary brain tumors in a large national cohort from the United States.
Methods: The National Cancer Database (NCDB) was queried for newly diagnosed primary brain tumors (2004–2014) in adult patients aged 18 and older receiving proton or photon radiotherapy. Clinical features, patient demographics and treatment parameters were extracted. Differences between groups were assessed using multivariable logistic regression analysis.
Results: In total, 73,073 patients were analyzed (n = 72,635 [99.4%] photon therapy, n = 438 [0.6%] proton therapy). On multivariable analysis of photon versus proton therapy, several factors predicted for receipt of proton therapy, including younger age (p = .041), highest income quartile (p = .007), treatment at academic institutions (p < .001), in regional facilities outside the Midwest/South (p < .001), diagnosis in more recent years (p = .003), fewer comorbidities (p < .001) and non-glioblastoma histology (p < .001).
Conclusions: There are several significant socioeconomic variables that influence receipt of proton therapy for primary brain tumors. Although not implying causation, the socioeconomic findings discovered herein should be taken into account when delivering cancer care to all patients.
Introduction
One of the fundamental and evolving themes of radiation therapy (RT) is to develop the increasingly selective delivery of radiation to tumor cells, in turn improving the therapeutic ratio of treatment [Citation1]. Since the discovery of the clinical usefulness of protons by Robert R. Wilson in 1946 [Citation2], over 149,000 patients have been treated with protons worldwide [Citation3], and the number of clinical proton therapy facilities continues to increase. Currently, there are nearly 30 active facilities and over 10 facilities under construction or in development in the United States alone [Citation4]. There is a growing interest in proton beam therapy (PBT) due to the potential for a more conformal dose distribution compared with more traditional photon therapy, with the majority of energy being deposited at the end of the proton’s Bragg’s peak [Citation1].
PBT has been increasingly utilized to treat a variety of cancers [Citation5–Citation18]; however, it has been shown to reduce unintended radiation exposure to normal tissue in most cases, its clinical advantages over photon therapy are still debated [Citation3]. Previous studies have analyzed the application of proton and photon therapy to pediatric medulloblastoma patients [Citation4], but there is a paucity of data regarding the influence of socioeconomic factors and administration of either treatment modality for other primary brain tumors in adults.
Although certain types of cancer have well-characterized associations with a patient’s socioeconomic status (SES), the association between primary brain tumors and SES has been less defined. There have been studies suggesting that higher SES [Citation19,Citation20] or white race [Citation21] is associated with higher incidences of glioma, which may be attributable to more readily available access to health care. A 2001 study found an increased risk of certain types of brain cancer within the elderly, Caucasians, men and people living in urban/metropolitan areas [Citation22], particularly regarding glioblastoma (GBM), oligodendroglioma, anaplastic astrocytoma, medulloblastoma and mixed glioma.
A study by Shen et al. analyzed the application of photon and proton therapy in pediatric patients [Citation23], suggesting correlations between delivered therapy and SES. In addition, certain demographic factors such as race, age and marriage status were demonstrated to have a negative impact on the utilization of RT in GBM patients [Citation24]. A study by Sherwood et al. concluded significant race and insurance-related discrepancies in the RT and chemotherapy treatment of astrocytomas, possibly related to issues regarding access to surgical intervention [Citation25]. Another study concluded that glioma patients with higher SES were more likely to receive surgery, radiation and gross total resection (GTR), whereas those with a lower SES had shorter survival times [Citation26]. The same study concluded that when the parameters were calibrated for treatment received, lower SES had a reduced but still significant effect on survival rate. However, this topic is still debated, with one study concluding that SES has no predictive effect on prognosis in patients with newly diagnosed GBM [Citation27].
To the best of our knowledge, there remains limited data on the impact of sociodemographics on the modality of radiotherapy, namely proton versus photon therapy, in adult gliomas. This is the first large-volume investigation into the utilization of proton versus photon therapy in the treatment of adult primary brain tumors in the United States. We sought to analyze disparities and variations in patterns of care in the use of either modality.
Material and methods
The National Cancer Database (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society, which consists of de-identified information regarding tumor characteristics, patient demographics and patient survival for ∼70% of the US population [Citation28–Citation34]. The NCDB contains information not included in the Surveillance, Epidemiology and End Results database, including details regarding chemotherapy and RT dosage [Citation5,Citation6]. The American College of Surgeons and the CoC have not verified and are neither responsible for the analytic or statistical methodology used nor the conclusions drawn from these data by the investigators. As all patient information in the NCDB database is de-identified, this study was exempt from institutional review board evaluation.
Criteria for this study included patients with histologically confirmed, primary intracranial brain tumors: GBMs, medulloblastomas, ependymomas and mixed lower-grade gliomas (as defined by NCDB codes 9380, 9382, 9391, 9392, 9400, 9401, 9420, 9421, 9440, 9441, 9442, 9450, 9451, 9470, 9471, 9473, 9506) treated with photon therapy (codes 23–27, 31, 32) or proton therapy (code 40). In accordance with the variables in NCDB files, captured variables on each patient broadly included basic demographics, socioeconomic factors, facility characteristics and tumor parameters.
All statistical tests were performed using SAS software (Version 9.4; Cary, NC, USA). Comparisons between the treatment groups and patient characteristics were based on Pearson’s Chi-square test for categorical data and the Wilcoxon rank-sum test for continuous data. Both univariate and multivariate logistic regression analyses were performed to investigate the patient characteristics were associated with the utilization of proton versus photon therapy. The predictor variables with significant amounts of missing values were excluded in the multivariate models. The covariates that were significant on the univariate logistic regression analyses at a 0.05 alpha level were entered into the multivariate model. The least significant covariates were excluded at each step, and the process continued until the model contained significant covariates. Odds ratio (OR), p values, and 95% confidence intervals (95% CI) were presented. A p value less than .05 was used as a threshold for statistical significance.
Results
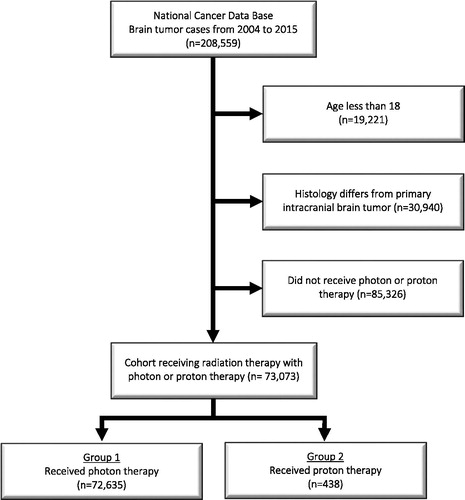
A complete flow diagram of patient selection is provided in . In total, 73,073 patients met study analysis criteria, with 72,635 (99.4%) receiving photon therapy and 438 (0.6%) receiving proton therapy. demonstrates the normalized temporal trends in treatment paradigm, with a steady rise in PBT after 2010.
Figure 2. Percentage of reported primary brain tumor proton cases out of all external beam radiation therapy (EBRT) cases of each diagnosis year.
displays notable clinical characteristics of the analyzed patients. After univariable analysis was performed (), multivariable analysis revealed that younger patient age (p = .041), income of greater than $63,000 (p = .007), treatment at academic centers (p < .001), a western facility location (p < .001), year of diagnosis 2010 or later (p = .003), a Charlson–Deyo score of 0 (p = .018) and non-GBM histology were independent predictors of proton therapy utilization ().
Table 1. Patient characteristics by modality.
Table 2. Univariate logistics regression analysis of factors predictive of proton therapy utilization.
Table 3. Multivariate logistics regression analysis of factors predictive of proton therapy utilization.
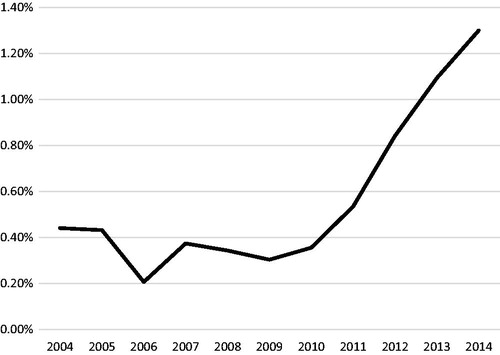
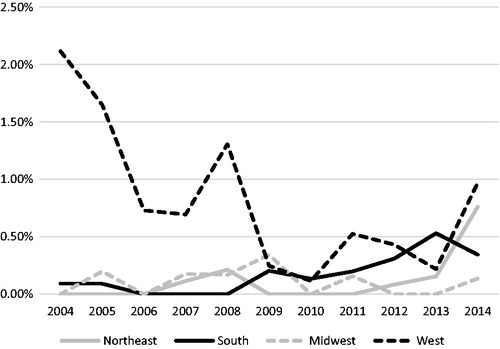
From 2004 to 2014, the relative use of PBT increased from just 0.44% of all patients to 1.30% in 2014 (). When displayed as the relative use of PBT per region (), there is a clear increased contribution of patients treated in the West, with 2.33% of patients receiving PBT in 2004, whereas in 2009 only 0.47% of patients in the West received PBT. By 2014, there was more or less an even distribution of the relative proportion of patients receiving PBT per region, with a slight predominance of relative cases utilizing PBT in the West and Northeast.
Figure 3. Percentage of reported primary brain tumor proton cases out of all external beam radiation therapy (EBRT) cases of each diagnosis year by region. Northeast (CT, MA, ME, NH, RI, VT, NJ, NY and PA); South (DC, DE, FL, GA, MD, NC, SC, VA, WV, AL, KY, MS, TN, AR, LA, OK and TX); Midwest (IL, IN, MI, OH, WI, IA, KS, MN, MO, ND, NE and SD); West (AZ, CO, ID, MT, NM, NV, UT, WY, AK, CA, HI, OR and WA).
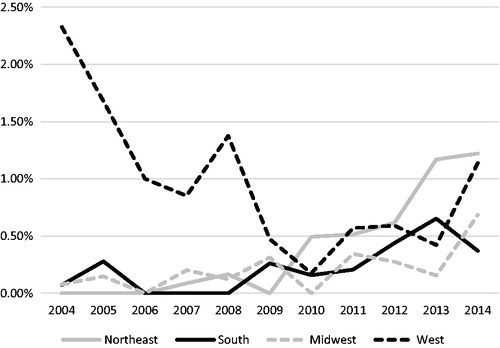
The same relative use trends were also investigated for GBMs, the largest contributor of patients accounting for 142 of 428 (32.4%) of the total cohort receiving PBT. From 2004 to 2014, the relative use of PBT for GBM patients increased from just 0.41% of all patients to 0.54% in 2014. However, this trend is unclear, as with the omission of the year 2014 there would be an overall downward trend of the use of PBT in GBM patients. When further analyzed by region (), the West predominated in early years, with 2.12% of patients receiving PBT in 2004, whereas in 2010 only 0.11% of patients in the West received PBT. By 2014, there was a more even distribution of relative GBM cases receiving PBT per region, with a slight prevalence of relative cases in the West and Northeast.
Discussion
These patterns of care analysis of the NCDB evaluating patients with primary brain tumors treated with photon or PBT demonstrates relatively few patients (0.6%) were treated with PBT over the past decade in the United States. This study suggests that receipt of PBT from 2004 to 2014 for adult primary brain tumors has at least doubled among NCDB reporting intuitions, whereas the trend for GBMs is less clear and may, in fact, be decreasing when the most recent year (2014) is omitted. The use of PBT is influenced by several variables, including younger age, fewer comorbidities, higher income, treatment at academic facilities and histology. These data have implications on payers, referral patterns and patients. Taken together, these findings indicate the challenge of, delivering high-quality care for cancer treatment for all patients in the United States.
Use of PBT was associated with younger patients compared with photon therapy, likely because of greater longevity in these patients. Proton therapy delivers less integral doses to surrounding tissue, which may be associated with secondary malignancies and other long-term consequences of tissue damage. This theoretical advantage may be more pronounced in younger patients and has been predicted in a variety of tumors, including thymomas [Citation35], seminomas [Citation36] and low-grade gliomas [Citation37]. In addition, proton therapy was predicted to decrease the risk of cardiac toxicity in breast cancer patients as compared to photon therapy [Citation38], demonstrating further potential in avoiding late side effects in younger patients. The association between proton therapy use and decreased Charlson–Deyo scores may be a confounding issue, as younger patients likely have less comorbidities compared with older patients.
The link between a later year of diagnosis and proton therapy may be correlated with the development and use of proton therapy in the United States. Proton therapy has been increasingly utilized over the years, and the rate at which centers are being built and expanded is increasing. Although the potential of proton therapy has been theorized since the 1940s [Citation2], its clinical use has been more recent, with the first proton therapy center in the United States opening in 1990. In terms of differences in regional utilization of PBT, this study demonstrated an increased relative proportion of patients treated with PBT in the West from 2004 to 2008 (). This trend is not unique for primary brain tumors, as it was also noted for in a recent NCDB pattern of care study for external beam radiotherapy of the prostate [Citation39]. The dominant relative utilization of PBT in the West receded in recent years, with the Northeast contributing the largest relative proportion of primary brain tumors treated with PBT in 2014 (the latest year provided by the NCDB for analysis at the time of this publication), which may be due to the high density of proton therapy centers in the Northeast. However, the increased trend for utilization of protons in the West and Northeast in this study is not without bias, as many proton therapy facilities are associated with academic centers that may have a higher propensity to be logged in the NCDB. Of the nearly 30 centers currently in use in the United States as of the writing of this manuscript, over half began operating after 2010 [Citation40]. Correspondingly, it is not surprising that this study demonstrated the relative utilization of PBT per year to be increasing steadily in this timeframe.
GBMs, which are known for in-field failures after RT, are also less likely to be treated with proton therapy. There has been little benefit to dose escalation in this disease [Citation41]; additionally, glioblastoma is traditionally treated with large fields, and the potential advantages of proton therapy to decrease treatment-related toxicities in the long term are not as likely to have a significant impact in these patients who have a relatively poor prognosis compared with other tumor types. Of the 51,975 GBM patientss treated with photon or proton therapy, only 142 patients (0.27%) received PBT. Although there was clearly a trend of increasing relative proportion for all patients receiving PBT in the years 2010 and later (), the trend in the subset of GBM is less clear (). First, 0.41% of GBM patients received PBT in the years 2004–2005, with each subsequent year failing to demonstrate a higher relative number. Indeed, there was an overall decreasing trend in the utilization of in subsequent years with the exception of an isolated value of 0.54% in the year 2014. When analyzed by the relative proportion of GBM patients receiving PBT by region (), perhaps the bimodal-like distribution in can be explained by the large proportion of patients treated with protons in the West initially, with a steadily decreasing trend thereafter until 2011. Of note, the average proportion for patients treated with GBMs in any region never reached above 1% after the year 2008, including the West, further suggesting overall decreased utilization of PBT in GBM patients. Nevertheless, the use of PBT should be questioned in the absence of prospective data demonstrating superiority over traditional photon beam therapy in the treatment of GBM.
Figure 4. Percentage of reported glioblastoma proton cases out of all external beam radiation therapy (EBRT) cases of each diagnosis year.
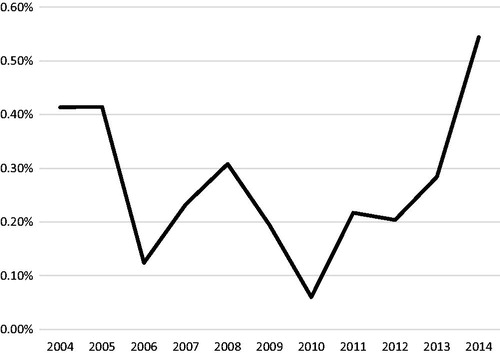
Figure 5. Percentage of reported glioblastoma proton cases out of all external beam radiation therapy (EBRT) cases of each diagnosis year by region. Northeast (CT, MA, ME, NH, RI, VT, NJ, NY and PA); South (DC, DE, FL, GA, MD, NC, SC, VA, WV, AL, KY, MS, TN, AR, LA, OK and TX); Midwest (IL, IN, MI, OH, WI, IA, KS, MN, MO, ND, NE and SD); West (AZ, CO, ID, MT, NM, NV, UT, WY, AK, CA, HI, OR and WA).
The advent of the WHO staging system update in 2016 underscored the importance of molecular subtyping, as genotype can now trump histological phenotype in staging [Citation42]. In fact, a large subset of tumors is now defined based on presence or absence of 1p/19q codeletion and IDH mutation, which one could largely expect the diagnosis of anaplastic oligoastrocytomas to largely disappear [Citation43]. Of the 2527 oligoastrocytoma and anaplastic oligoastrocytoma patients in this study, only 537 (21.3%) had information on 1p/19q deletion. However, the frequency of molecular information in regards to 1p/19q codeletion improved in the latter years, with 528 of 1242 of cases (42.5%) containing this information in the years 2010 or later, suggesting improvement of coding in more recent years. In fact, dropping 2004–2009 from the dataset only loses 9 patients who had 1p/19q codeletion status for the subset of oligoastrocytoma and anaplastic oligoastrocytoma patients in this study. Isocitrate dehydrogenase (IDH) status is not tabulated in the NCDB. Therefore, this study was unable to tease out differences in utilization of proton therapy for WHO II versus WHO III gliomas in the modern era due to inherent limitations related to the coding of molecular status within the NCDB. Accordingly, future studies utilizing the NCDB wishing to investigate patterns of care in the molecular era should be aware of the paucity of molecular information before the year 2010.
Patients with high incomes were more likely to receive proton therapy, likely related to the cost of proton therapy for both the patient and the institution. A 2013 study indicated that the median amount reimbursed by Medicare was $32,428 for proton therapy, compared with $18,575 for intensity-modulated radiation therapy (IMRT) [Citation44]. As such, certain non-Medicare insurances may be less likely to cover PBT. For pediatric patients, insurance policies have previously denied coverage over a certain age, or in specific pediatric tumors [Citation45]. In adults, insurance coverage is non-uniform, with private insurance agencies largely being involved with the assessment of PBT coverage [Citation46]. However, it has been argued that previous models of cost-effectiveness are not applicable to the current landscape of PBT given the changes in the field in the last decade, including the expansion of centers across the United States and changes in technology [Citation47–49]. Additional factors explaining the demonstrated income disparity in treatment modalities may include an association between proton therapy center location and local income level, though this possible association must be further investigated. Certain confounding variables may also have an impact; though insurance was not was not found to be significant on multivariate analysis, it was significant on univariate analysis and may be related to income level. In addition, younger patients, a demographic less likely to receive Medicare, are also more likely to receive proton therapy. The NCDB provides a wealth of data for investigating disparities in photon and proton therapy utilization. However, this study has limitations. As this study investigates several primary brain tumors, it is unable to divulge more specifically how discrepancies in treatment modalities play out in each type of brain tumor, nor would a survival analysis be prudent given the vast differences in prognosis. Therefore, this study did not examine how socioeconomic variables, histology and treatment affected overall survival as it would not be suited for such analysis due to the heterogeneity of brain tumor types. Next, not all CoC facilities contribute data to the NCDB, which may limit the database’s representation of the whole population. Referral patterns to proton facilities of these patients may also not necessarily represent those of other parts of the United States or the world. In addition, state insurance coverage of various radiation modalities varies, further modifying how it factors into the spread of modalities. Coverage is also dynamic over time, a factor that cannot be assessed by this work. In addition, the most recent year of data captured by the NCDB is 2014, which may misrepresent contemporary trends from 2015 to 2018; because these trends could be different from prior years given the exponential rise in proton therapy, further analyses in the future are recommended. Lastly, although the NCDB is the largest known comprehensive database in the United States, the estimated 30% of the relevant population that does not contribute cases could skew the data in several ways; because academic facilities (which are more likely to have proton centers) are more likely to report data to the NCDB than small, community centers without proton facilities, the patterns of care herein would naturally be tilted in favor of proton therapy and those receiving therapy at larger, academic centers.
This study suggests that receipt of PBT for adult primary brain tumors is rising over time, and is influenced by several variables, including younger age, fewer comorbidities, higher income, treatment at academic facilities and histology. These data have implications on payers, referral patterns and patients. Taken together, these findings add to our known knowledge of the challenge of, delivering equality of health care for cancer treatment for all patients in the United States.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kooy HM, Delaney TF. Proton and charged particle radiotherapy. Philadelphia (PA): Lippincott Williams & Wilkins, 2008.
- Wilson RR. Radiological use of fast protons. Radiology. 1946;47:487–491.
- Jermann M. Particle therapy patient statistics [Particle Therapy Co-Operative Group website] Patient statistics per end of 2016 [cited 2018 May 15]. Available from: https://www.ptcog.ch/index.php/patient-statistics.
- Proton Therapy Centers. [The National Association for Proton Therapy website] [cited 2018 May 15]. Available from: http://www.proton-therapy.org/map.htm.
- Adeberg S, Harrabi SB, Verma V, et al. Treatment of meningioma and glioma with protons and carbon ions. Radiat Oncol. 2017;12:193.
- Adeberg S, Bernhardt D, Harrabi SB, et al. Sequential proton boost after standard chemoradiation for high-grade glioma. Radiother Oncol. 2017;125:266–272.
- Verma V, Rwigema JC, Malyapa RS, et al. Systematic assessment of clinical outcomes and toxicities of proton radiotherapy for reirradiation. Radiother Oncol. 2017;125:21–30.
- Chang JY, Verma V, Li M, et al. Proton beam radiotherapy and concurrent chemotherapy for unresectable stage III non–small cell lung cancer: final results of a phase 2 study. JAMA Oncol. 2017;3:e172032.
- Rwigema JC, Verma V, Lin L, et al. Prospective study of proton-beam radiation therapy for limited-stage small cell lung cancer. Cancer. 2017;123:4244.
- Verma V, Iftekaruddin Z, Badar N, et al. Proton beam radiotherapy as part of comprehensive regional nodal irradiation for locally advanced breast cancer. Radiother Oncol. 2017;123:294–298.
- Lin SH, Merrell KW, Shen J, et al. Multi-institutional analysis of radiation modality use and postoperative outcomes of neoadjuvant chemoradiation for esophageal cancer. Radiother Oncol. 2017;123:376–381.
- Verma V, Lin SH, Simone CB, et al. Clinical outcomes and toxicities of proton radiotherapy for gastrointestinal neoplasms: a systematic review. J Gastrointest Oncol. 2016;7:644.
- Verma V, Moreno AC, Lin SH. Advances in radiotherapy management of esophageal cancer. J Clin Med. 2016;5:91.
- Verma V, Simone CB, Wahl AO, et al. Proton radiotherapy for gynecologic neoplasms. Acta Oncol. 2016;55:1257–1265.
- Chuong MD, Hallemeier CL, Jabbour SK, et al. Improving outcomes for esophageal cancer using proton beam therapy. Int J Radiat Oncol Biol Phys. 2016;95:488–497.
- Verma V, Shah C, Mehta MP. Clinical outcomes and toxicity of proton radiotherapy for breast cancer. Clin Breast Cancer. 2016;16:145–154.
- Merchant TE, Farr JB. Proton beam therapy: a fad or a new standard of care. Curr Opin Pediatr. 2014;26:3–8.
- Kopecky AS, Khan AJ, Pan W, et al. Outcomes and patterns of care in a nationwide cohort of pediatric medulloblastoma: factors affecting proton therapy utilization. Adv Radiat Oncol. 2017;2:588–596.
- Porter AB, Lachance DH, Johnson DR. Socioeconomic status and glioblastoma risk: a population-based analysis. Cancer Causes Control. 2015;26:179–185.
- Plascak JJ, Fisher JL. Area-based socioeconomic position and adult glioma: a hierarchical analysis of surveillance epidemiology and end results data. PLoS One. 2013;8:e60910.
- Demers PA, Vaughan TL, Schommer RR. Occupation, socioeconomic status, and brain tumor mortality: a death certificate-based case-control study. J Occup Med. 1991;33:1001–1006.
- Deorah S, Lynch CF, Sibenaller ZA, et al. Trends in brain cancer incidence and survival in the United States: surveillance, epidemiology, and end results program, 1973 to 2001. Neurosurg Focus. 2006;20:E1.
- Shen CJ, Hu C, Ladra MM, et al. Socioeconomic factors affect the selection of proton radiation therapy for children. Cancer. 2017;123:4048–4056.
- Aizer AA, Ancukiewicz M, Nguyen PL, et al. Underutilization of radiation therapy in patients with glioblastoma: predictive factors and outcomes. Cancer. 2014;120:238–243.
- Sherwood PR, Dahman BA, Donovan HS, et al. Treatment disparities following the diagnosis of an astrocytoma. J Neurooncol. 2011;101:67–74.
- Deb S, Pendharkar AV, Schoen MK, et al. The effect of socioeconomic status on gross total resection, radiation therapy and overall survival in patients with gliomas. J Neurooncol. 2017;132:447–453.
- Kasl RA, Brinson PR, Chambless LB. Socioeconomic status does not affect prognosis in patients with glioblastoma multiforme. Surg Neurol Int. 2016;7:282.
- Bilimoria KY, Bentrem DJ, Stewart AK, et al. Comparison of Commission on Cancer–approved and–nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27:4177–4181.
- Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690.
- Moreno AC, Verma V, Hofstetter WL, et al. Patterns of care and treatment outcomes of elderly patients with stage I esophageal cancer: analysis of the National Cancer Data Base. J Thorac Oncol. 2017;12:1152–1160.
- Stahl JM, Corso CD, Verma V, et al. Trends in stereotactic body radiation therapy for stage I small cell lung cancer. Lung Cancer. 2017;103:11–16.
- Verma V, McMillan MT, Grover S, et al. Stereotactic body radiation therapy and the influence of chemotherapy on overall survival for large (≥5 centimeter) non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;97:146–154.
- Lerro CC, Robbins AS, Phillips JL, et al. Comparison of cases captured in the national cancer data base with those in population-based central cancer registries. Ann Surg Oncol. 2013;20:1759–1765.
- Mohanty S, Bilimoria KY. Comparing national cancer registries: the National Cancer Data Base (NCDB) and the surveillance, epidemiology, and end results (SEER) program. J Surg Oncol. 2014;109:629–630.
- Vogel J, Lin L, Litzky LA, et al. Predicted rate of secondary malignancies following adjuvant proton versus photon radiation therapy for thymoma. Int J Radiat Oncol Biol Phys. 2017;99:427–433.
- Simone CB, Kramer K, O’Meara WP, et al. Predicted rates of secondary malignancies from proton versus photon radiation therapy for stage I seminoma. Int J Radiat Oncol Biol Phys. 2012;82:242–249.
- Harrabi SB, Bougatf N, Mohr A, et al. Dosimetric advantages of proton therapy over conventional radiotherapy with photons in young patients and adults with low-grade glioma. Strahlenther Onkol. 2016;192:759–769.
- Stick LB, Yu J, Maraldo MV, et al. Joint estimation of cardiac toxicity and recurrence risks after comprehensive nodal photon versus proton therapy for breast cancer. Int J Radiat Oncol Biol Phys. 2017;97:754–761.
- Amini A, Raben D, Crawford ED, et al. Patient characterization and usage trends of proton beam therapy for localized prostate cancer in the United States: a study of the National Cancer Database. Urol Oncol. 2017;35:438–446.
- Particle therapy facilities in operation [Particle Therapy Co-Operative Group website] April 2018 [cited 2018 May 15]. Available from: https://www.ptcog.ch/index.php/facilities-in-operation
- Chan JL, Lee SW, Fraass BA, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol. 2002;20:1635–1642.
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820.
- Wesseling P, Capper D. WHO 2016 classification of gliomas. Neuropathol Appl Neurobiol. 2018;44:139–150.
- Yu JB, Soulos PR, Herrin J, et al. Proton versus intensity-modulated radiotherapy for prostate cancer: patterns of care and early toxicity. J Natl Cancer Inst. 2013;105:25–32.
- Ojerholm E, Hill-Kayser CE. Insurance coverage decisions for pediatric proton therapy. Pediatr Blood Cancer. 2018;65:e26827.
- Shah A, Ricci KI, Efstathiou JA. Beyond a moonshot: insurance coverage for proton therapy. Lancet Oncol. 2016;17:559–561.
- Verma V, Shah C, Rwigema JC, et al. Cost-comparativeness of proton versus photon therapy. Chin Clin Oncol. 2016;5:56.
- Verma V, Mishra MV, Mehta MP. A systematic review of the cost and cost-effectiveness studies of proton radiotherapy. Cancer. 2016;122:1483–1501.
- Verma V, Simone CB, Mishra MV. Quality of life and patient-reported outcomes following proton radiation therapy: a systematic review. J Natl Cancer Inst. 2018;110:341–353.