 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background: Occupational exposure has been identified as the most important risk factor for bladder cancer second to smoking. The objective of this study was to estimate the occupational variation in risk of bladder cancer that is not attributable to smoking.
Material and methods: In the Nordic Occupational Cancer study (NOCCA), 111,458 cases of bladder cancer and 208,297 cases of lung cancer cases were observed among men in Denmark, Finland, Iceland, Norway and Sweden during 1961–2005. Relative smoking prevalence in an occupation was estimated based on standardized incidence ratio (SIR) for lung cancer in the given occupation. Crude and smoking-adjusted SIRs with 95% confidence intervals (CI) for bladder cancer were calculated for each occupation.
Results: The smoking-adjusted SIR for most of the occupations was closer to 1.00 than the unadjusted SIR. The highest statistically significant smoking-adjusted SIRs were observed among chimney sweeps (SIR 1.29, 95% CI 1.05–1.56), waiters (1.22, 1.07–1.38) hairdressers (1.14, 1.02–1.26), cooks and stewards (1.12, 1.01–1.25), printers (1.11, 1.04–1.18) and seamen (1.09, 1.03–1.14).
Conclusions: Smoking is a strong risk factor for bladder cancer but there may also be other factors in some specific occupations in addition to smoking. The occupational variation in risk of bladder cancer is small when adjusted for smoking, but risk increasing factors are indicated in some occupations.
Introduction
Tobacco-smoking and occupational exposure, are the leading risk factors for bladder cancer. It has been estimated that occupational exposure may account for as many as 20% of all the bladder cancer cases in industrialized countries, and it is the second most important risk factor after smoking [Citation1,Citation2]. Workplace exposure to chemical carcinogens such as aromatic amines and polycyclic aromatic hydrocarbons (PAH) has been associated with increased risk of bladder cancer among painters, printers, drivers, hairdressers, launderers, and miners [Citation2,Citation3]. Bladder cancer is more common in men than in women, and the risk increases with age. In the Nordic countries, it is the fifth most common cancer in men. Age-standardized incidence rates increased until 1990, with the highest incidence in Denmark and the lowest in Finland [Citation4]. There have been temporal changes in exposure to workplace carcinogens in the Nordic countries. According to the FINJEM, exposure to carcinogens such as benzene, benzo(a)pyrene, and asbestos have substantially decreased in Finland from 1950 to 2008 [Citation5]. Similarly, a Danish study reported decreased exposure to trichloroethylene by 4% from 1947 to 1964 and by 15% from 1964 to 1989 [Citation6]. Another Danish study observed similar decreased trend of exposure to styrene, toluene and xylene over the period of 1955–1988 [Citation7]. A Norwegian study reported a decreasing trend in exposure to polycyclic aromatic hydrocarbons among the Norwegian industry workers [Citation8]. Overall, the carcinogenic exposures have decreased in last 20 years in the Nordic and in some other industrialized countries [Citation9,Citation10].
In the Nordic countries, Lung cancer incidence in Finnish men increased until 1970 and decreased sharply after that [Citation4]. In Denmark and Sweden, the incidence started to decrease after 1980 and in Iceland and Norway after 2000 [Citation4]. Smoking was assumed to be a crucial risk factor behind the occupational variations in both bladder and lung cancers [Citation11,Citation12]. However, the studies do not have information on occupation-specific smoking habits, and therefore we cannot separate the roles of smoking and occupational exposures. Methods for control for tobacco-smoking in the absence of individual-level smoking information have been discussed over a long period [Citation13–17]. The suggested methods are based on combined information on smoking habits from numerous sources or from historical estimates of the effect of the confounders.
Epidemiological evidence of the causal association between cigarette-smoking and lung cancer was established around the 1950s. The International Agency for Research on Cancer (IARC) and an international working group of experts recognized the causal relationship between tobacco-smoking and lung cancer in 1985 [Citation18]. Since then, a large number of studies have reported significant association between tobacco-smoking and the risk of lung cancer. Taking advantage of the convincing evidence of a strong association between smoking and lung cancer risk, we can consider the incidence of lung cancer in a specific occupational category to be a proxy for smoking prevalence in that occupation, unless there are other strong lung cancer carcinogens in the occupation. In our large population-based study, which included all working-age men in five Nordic countries, we assessed occupational variations in the risk of bladder cancer, adjusted with a proxy for smoking based on the incidence of lung cancer.
Material and methods
This study utilized the Nordic Occupational Cancer Study (NOCCA), comprising up to 45 years of cancer incidence data by occupational categories. The cohort consists of 14.9 million people from all five Nordic countries (Denmark, Finland, Iceland, Norway, and Sweden), aged 30 to 64 years, who participated in one or more population censuses in 1960, 1970, 1980/81, or 1990 and were living in the country on 1 January after the census year. The census information on occupation for each individual was obtained through the questionnaires in the censuses, which were centrally coded and computerized in the national statistics offices. The original occupational codes were then reclassified into 53 different categories. Details of the NOCCA study have been described elsewhere [Citation12]. Data on incident cancer cases were obtained from the national cancer registers in each of the Nordic countries. The follow-up started on 1 January after the earliest available census, and ended at death or at the end of the year 2003–2005 (). The registration of new cancer cases is based on the information from clinical and pathological departments, general practitioners, and private clinics. The largest incomparability between the Nordic countries is the absence of death certificates in Sweden, which has been estimated to cause a loss of up to 4% of all cancer cases [Citation19]. Due to lack of information on smoking habits in all occupational categories in all Nordic countries over the study period, we estimated a proxy smoking prevalence in the occupational groups from the lung cancer incidence in the NOCCA data set. This study was undertaken with linkage of individual records based on the unique personal identity codes used in all the Nordic countries.
Table 1. Demographic variables of the study populations of bladder and lung cancer in Nordic countries.
The results are presented as standardized incidence ratios (SIRs), i.e., ratios of observed to expected numbers of cases. In this study, we present SIRs for bladder cancer, while SIRs for lung cancer are used as proxies for smoking prevalence in an occupational category. For each country, gender, and occupational category, the observed number of cancer cases and person years was stratified into eight five-year attained age categories (30–34, 35–39…, 85+ years) and nine five-year calendar periods (1961–1965…, 2001–2005). For the SIRs for bladder cancer that were not adjusted for smoking, the expected number of cases in each country was calculated by multiplying the number of person years in each stratum by the respective national bladder cancer incidence rate.
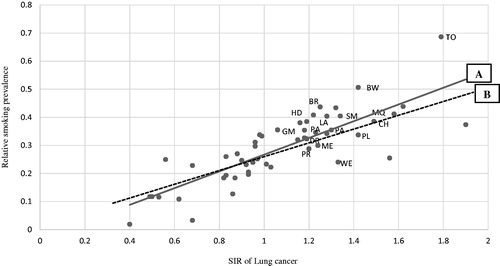
For smoking-adjusted SIRs (SIRadj), the expected number was corrected by multiplying the original expected number by the product of the proxy smoking prevalence value and the relative risk (RR) estimate for bladder cancer due to smoking in each occupational category [Citation17,Citation20]. For the purposes of analysis, we calculated the continuous RR based on smoking prevalence. Smoking prevalence was estimated based on the SIR for lung cancer (). The estimation of smoking prevalence (Y) was based on linear regression (Y = α + βX), where X is the SIR of lung cancer, α = Y − intercept value of smoking prevalence, and β = slope of the line (). From the figure, α = 0.4 and the tangent function of regression line β = 0.28 approximately. The SIR for lung cancer is about 0.2 when smoking prevalence is zero, and about 1.8 when smoking prevalence is 0.5 (Line A in ). It shows the risk increases by nine times with a five time increase in smoking prevalence. Hence, if we assume that the RR for bladder cancer due to smoking is one third of lung cancer, the RR for bladder cancer increases by 0.18 with every 10% unit increase in smoking prevalence. Analyses were performed in two different models. In Model 1, we included all 53 occupational categories (Line A in ). In Model 2, we excluded occupations with smoking-adjusted SIRs for lung cancer markedly higher than 1.0 according to an earlier Norwegian study [Citation14], i.e., miners and quarry workers, drivers, smelting workers, mechanics, plumbers, welders, painters, bricklayers, printers, beverage workers, tobacco workers, glass makers, packers, hairdressers, launderers and gardeners (Line B in ). The lung cancer rate for these occupations may reflect other major risk factors in addition to smoking. Exact 95% confidence intervals (CIs) of the SIRs were defined based on Poisson distribution. We used two-sided tests for a statistical significance level of 0.05. The ethical committees and data inspection boards in each of the Nordic countries approved the study.
Figure 1. Smoking prevalence on the 53 occupational categories estimated based on standardized incidence ratios (SIR) of lung cancer in Nordic males. Regression line A: Estimation based on all 53 occupations (y = α + βx). Regression line B: Estimation based only on 37 occupations excluding occupations with probable strong occupation-related risk factors other than smoking; miners and quarry workers, drivers, smelting workers, mechanics, plumbers, welders, painters, bricklayers, printers, beverage workers, tobacco workers, glass makers, packers, hairdressers launderers and gardeners (y = α + βx).
Results
The study included 111,458 cases of bladder cancer and 208,297 cases of lung cancer, observed during a total follow-up time of 184.9 million person years (). The highest number of cases was from Sweden and the lowest from Iceland. The estimated smoking prevalence varied across the occupational categories. The correlation coefficient value (r 0.81, 0.78) shows the dose-response relationship of lung cancer incidence due to smoking ().
The highest statistically significant unadjusted SIRs were observed for chimney sweeps (1.49, 1.21–1.80), waiters (1.42, 1.25–1.61), hairdressers (1.30, 1.17–1.45), cooks and stewards (1.20, 1.07–1.34), and printers (1.18, 1.11–1.26). The SIRs came closer to 1.00 for all occupations after adjustment with the proxy for smoking. The adjusted results from Models 1 and 2 were similar. Therefore, we chose to present the results from Model 1 ( and ).
Figure 2. Original (square) and smoking adjusted (model 1, circle) standardized incidence ratios (SIR) for 53 occupational categories in Nordic males.
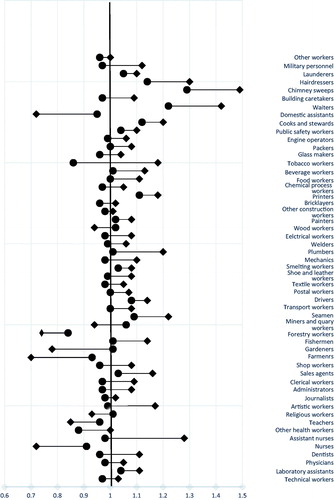
Table 2. Observed number of bladder cancer cases (Obs), standardized incidence ratio (SIR), 95% confidence interval (95% CI) with proxy smoking adjusted from lung cancer in occupations in Nordic males.
Table 3. Observed number of bladder cancer, standardized incidence ratio (SIR), 95% confidence interval (95% CI) with proxy smoking adjusted from lung cancer in occupations with smoking-adjusted SIR from Model 1 (SIRadj1) > 1.05 in Nordic males, by country.
SIRadj1 was >1.10 for chimney sweeps (1.29, 95% CI 1.05–1.56), waiters (1.22, 1.07–1.38), hairdressers (1.14, 1.02–1.26), cooks and stewards (1.12, 1.01–1.25), and printers (1.11, 1.04–1.18). A small but significant increased risk was also observed among seamen and drivers, while a similar non-significant risk was observed among laboratory assistants, miners and quarry workers, public safety workers, and launderers (). The SIRadj1 for bladder cancer appeared to be similarly elevated across the Nordic countries, e.g., for drivers, printers, and hairdressers. For cooks and stewards, waiters, and chimney sweeps, the SIRadj1 increased in some countries but decreased in others.
Discussion
This is the largest study to explore the association between occupation and risk of bladder cancer adjusted for smoking. Chimney sweeps, waiters, hairdressers, cooks and stewards and printers had the highest risk of bladder cancer after adjusting for smoking habits. The highest estimated prevalence of smoking was observed in Denmark and the lowest in Sweden (data not shown).
The most pronounced increased risk of bladder cancer in Nordic males was observed in chimney sweeps. A systematic review and meta-analysis of modifiable risk factors for the prevention of bladder cancer from 1995 to 2015 observed that chimney sweeps were among the occupations with the highest risk of bladder cancer (RR 1.53, 1.30–1.81) [Citation21]. Another meta-analysis of 263 eligible articles published to June 2015 observed a similar high risk among chimney sweeps [Citation22]. These studies observed that persons exposed to chemical solvents such as PAH and aromatic amines are at higher risk of bladder cancer. However, not all the studies included in this study were adjusted for smoking. Chimney sweeps are exposed to high levels of soot rich in PAH, which predominantly causes lung and bladder cancers [Citation23–25].
Waiters were observed to be another high-risk group in our study. Similar risks were observed in different studies. A systematic review and meta-analysis observed that waiters had among the highest risks (RR 1.43, 1.34–1.52) of bladder cancer in occupational cancer studies after adjusting for smoking status [Citation22]. A case control study in the United States observed an overall (OR 2.84, 1.05–7.72) risk of bladder cancer following exposure for more than 10 years among those employed as waiters [Citation26]. A case control study from Italy [Citation27] and a cohort study from Sweden [Citation28] observed similar increased risks. It should be noted that the data from the Swedish study markedly overlap with the data in our study. Waiters are mainly exposed to both active and passive smoking, and possibly to other workplace carcinogenic substances such as PAH [Citation29]. Legislation regarding smoke-free restaurants and other public places was implemented in the Nordic countries after the period of our study, in 2005. Future studies are needed to show whether the small excess in the risk of bladder cancer will disappear.
In our study, hairdressers were at elevated risk of bladder cancer. In a meta-analysis by Harlings et al., a summary risk ratio (SRR) of 1.70 (95% CI 1.10–2.88) for bladder cancer was observed among hairdressers [Citation30]. The study also observed an increase in SRR with an increase in the duration of employment, and observed no differences in the estimates of crude and smoking-adjusted risks. Another meta-analysis observed a pooled RR of 1.30 (95% CI 1.20–1.42) for bladder cancer among hairdressers [Citation31]. The excess in this study was restricted to exposures before the ban in 1970 on the carcinogenic aromatic amines used in hair dyes. Hairdressers have historically been exposed to aromatic amines such as benzidine, toluidine and aromatic nitrous compounds, and exposure to these chemical compounds has been linked to an increased risk of bladder cancer [Citation32]. Long-term exposure of >10 years has been associated with greater risk of bladder cancer [Citation31,Citation33]. A study among Finnish hairdressers showed that there was a marked overall excess risk of cancer until the mid-1970s, but almost no excess after that; this was interpreted as a consequence of a rapid decrease in exposure to carcinogenic agents [Citation34]. Following the ban on carcinogenic substances in the European Union, some recent studies have reported an excess bladder cancer risk, but the risk is due to the use of such banned substances even after legislation, leading to the potential for ongoing exposure [Citation35]. A separate NOCCA study on a period-specific stratified analysis from 1961 to 2005 observed a decreased risk among hairdressers [Citation36].
Increased risk of bladder cancer was associated with employment as cooks and stewards in our study. These workers are commonly exposed to environmental tobacco smoke and other workplace chemical carcinogens associated with the risk of bladder cancer [Citation22]. Two reviews, by Cumberbatch et al. and Al-Zalabani et al., reported the same statistically significant increased pooled relative risk (RR 1.15, 1.08–1.22) of bladder cancer among those exposed as cooks and stewards [Citation21,Citation22]. A higher relative excess was observed in males.
Printers were another high-risk group for bladder cancer observed in the study. A meta-analysis observed a significant pooled relative risk (RR 1.23, 1.17–1.30) among printers [Citation22]. Another meta-analysis of case control studies from six European countries observed up to 80% greater risk of bladder cancer among those exposed as printers compared with the general population (OR 1.81, 95% CI 1.03–3.17) [Citation3]. Printers are exposed to pigments and solvents (vehicles) that are carcinogenic with a risk of bladder cancer [Citation37]. The IARC has classified occupational exposures in printing processes as possibly (group 2B) carcinogenic to humans [Citation38].
In the absence of direct information on their occurrence or the magnitude of the population under study, it is possible to estimate the impact of confounding using indirect (proxy) measures [Citation16]. In our study, we observed that the smoking-adjusted SIRs came closer to 1.0 for all occupations after adjustments with the proxy for smoking, but the adjustment did not change the direction of the risk estimate. This is in line with the findings of other studies that also observed that adjustment for smoking did not normally change the direction of the risk estimate [Citation13–16,Citation39]. Consistency across the country-specific risk estimate observations for a given occupation might help to separate true associations from chance findings. In this type of large cohort study, it has been estimated that either systematic or chance differences in unmeasured lifestyle factors such as smoking will not confound the risk by more than 20% [Citation16,Citation39], and we can safely conclude that the residual confounding due to smoking in our smoking-adjusted risk estimates is smaller. Hence, it is very unlikely that occupational variations in the risk of bladder cancer will affect the risk estimate due to smoking alone. Exposure to chemical agents, such as PAH, aromatic amines, and other workplace carcinogens is associated with the risk of bladder cancer [Citation22].
In the Nordic countries, smoking prevalence corresponds well to the lung cancer incidence. This pattern of smoking prevalence and lung cancer incidence was reported in the national surveys separately in all the Nordic countries. According to the Norwegian national survey from 1965 to 1980, occupations with a high-risk of lung cancer had also a high level of smoking [Citation14]. In this study, Haldorsen et al. estimated a 10% increase in the proportion of smoking to increase the lung cancer incidence by 26% in men. Similarly, data from Finnish national surveys (FINJEM) [Citation40], Swedish national survey [Citation17] and Norwegian national survey [Citation14,Citation41] estimated the occupation specific prevalence of smoking and other lifestyle factors in different occupational categories. In all these studies, smoking was positively associated with both lung and bladder cancer across different occupational categories [Citation12]. Some of the most high-risk occupations included waiters, tobacco workers, seamen, miners and quarry workers, cooks and stewards, and transportation workers [Citation12,Citation14,Citation41]. The surveys included information on sex, year of birth, age at smoking, smoking status (current smoking, former smoking) average number of cigarettes per day for cigarette smokers and the proportion of smoking a pipe among current smokers to describe their smoking habits [Citation14,Citation17,Citation41]. These surveys had a high response rate and the validity of these data are considered to be of high standard [Citation19].
Limitations of the study include exposure misclassification. As the individuals’ occupational categories were based on occupations held in the year before the first available census, the data did not provide information on job changes across the entire working career. Misclassification of the exposure estimate would bias the observed effect towards null. However, occupational stability, e.g., in Finland, was high in the early decades, and turnover was low in the older male population [Citation42]. Therefore, exposure misclassification in this NOCCA study should be relatively small. Nonetheless, we cannot rule out the possibility of residual confounding. The occupational titles were categorized into 53 broader categories to reach sufficient numbers of cancer cases in each category. Some of the occupational categories used in the study are heterogeneous and may hide more specific subcategories with occupation-specific risk factors. A similar limitation also concerns the use of smoking prevalence in an occupational category as a proxy for lifelong smoking habits for all men in that occupation. It is known from Finnish survey data that although the prevalence of smoking in men has decreased strongly in all socio-economic categories, the relative difference between categories has not changed [Citation43,Citation44]. If we assume that the latency time from smoking to cancer outcome is similar for lung cancer and bladder cancer, the use of SIRs for lung cancer to adjust bladder cancer SIRs for a similar period should take care of the complex lag-time issue.
The NOCCA study is the largest ever conducted on occupational cancer incidence, with virtually full coverage of cancer cases in the Nordic countries, and with large numbers of bladder and lung cancer cases. The occupational codes used are of high accuracy [Citation12]. The completeness and accuracy of the Nordic cancer registers is of top quality in international rankings [Citation19]; even if there were some incompleteness in registration or diagnosis, this would not affect SIR estimates as long as the cancer registration and diagnosis followed similar procedures irrespective of the person’s occupation. The unique personal identity code, used for linkage between the census, vital status and cancer incidence data, is a highly reliable linkage tool [Citation19].
This study suggests that smoking is not the only factor causing variation in bladder cancer incidence between the occupational categories but carcinogenic workplace exposures may also play a role in some specific occupations such as chimney sweeps, waiters, hairdressers, cooks and stewards, printers and seamen. Occupational exposure pattern has changed over the study period in all Nordic countries, primarily after following the ban on the use of some carcinogenic substances, e.g., aromatic amines, at workplaces after the 1980s in the European Union. Similarly, other preventive measures were introduced as a consequence of an improved legislation, implementation of safety and hygiene programs, elimination or decreased production of hazardous chemical substances that are classified as risk to human health, and substitution with few carcinogens are some of the remarkable measures for decreased risk [Citation10]. Additionally, it is indeed a known fact that there is an increased risk of second smoking-related cancers in patients with first smoking-related cancer. In the NOCCA dataset also the second and higher primary malignancies are counted and therefore a person having e.g., head and neck cancer first and lung cancer later will contribute to lung cancer statistics.
Although there has been a decreasing trend in PAH exposure in the recent decades in the Nordic countries [Citation45], it is still a problem causing lung and bladder cancer. PAHs are important workplace carcinogens that occur in various forms such as benzo[a]pyrene and soot. A Swedish study covering all male chimney sweeps observed increased risk of bladder and lung cancer due to PAH (soot) exposure [Citation25]. Randem et al. reported similar increased risk of lung and bladder cancer due to PAH exposure among male asphalt industry workers in Denmark, Finland, Norway and Sweden [Citation45]. Parallel to the findings of these studies, chimney sweeps in our study was observed as the highest risk of bladder cancer and workplace exposure to PAH could be an important factor for increased risk. Globally, workplace PAH exposure has been reduced after the peak in the mid-1990s [Citation46]. The risk due to occupational carcinogens at workplace carcinogens such as PAH, asbestos, diesel engine exhaust is shifting towards the developing countries [Citation47].
In summary, occupation is evidently associated with the risk of bladder cancer, but most of the variation in bladder cancer risk observed between occupational categories appears to be due to smoking. After adjustment with a proxy for smoking prevalence, we observed an evident excess in bladder cancer risk only in some occupational categories, most of which are suspected of facing occupational exposures to chemical carcinogens of such high levels that they could add to the risk of bladder cancer. The large size of our study made it possible to reveal significant findings in rare occupations such as chimney sweeps and male hairdressers. Future research on occupational exposures to chemical compounds would better explain the risk variation between occupational categories.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Silverman DT, Levin LI, Hoover RN, et al. Occupational risks of bladder cancer in the United States: I. White men. J Natl Cancer Inst. 1989;81:1472–1480.
- Wilcox AN, Silverman DT, Friesen MC, et al. Smoking status, usual adult occupation, and risk of recurrent urothelial bladder carcinoma: data from The Cancer Genome Atlas (TCGA) Project. Cancer Causes Control. 2016;27:1429–1435.
- Kogevinas M, 't Mannetje A, Cordier S, et al. Occupation and bladder cancer among men in Western Europe. Cancer Causes Control. 2003;14:907–914.
- Association of the Nordic cancer registries, NORDCAN. Cancer incidence and mortality in the Nordic countries. 2017 version 8.0.2017. [cited 2018 Aug 6]. Available from: http://www-dep.iarc.fr/NORDCAN/english/testdb/frame.asp.
- Kauppinen T, Uuksulainen S, Saalo A, et al. Trends of occupational exposure to chemical agents in Finland in 1950-2020. Ann Occup Hyg. 2013;57:593–609.
- Raaschou-Nielsen O, Hansen J, Thomsen BL, et al. Exposure of Danish workers to trichloroethylene, 1947-1989. Appl Occup Environ Hyg. 2002;17:693–703.
- Jensen AA, Breum NO, Bacher J, et al. Occupational exposures to styrene in Denmark 1955-88. Am J Ind Med. 1990;17:593–606.
- Romundstad P, Haldorsen T, Rønneberg A. Exposure to PAH and fluoride in aluminum reduction plants in Norway: historical estimation of exposure using process parameters and industrial hygiene measurements. Am J Ind Med. 1999;35:164–174.
- Burstyn I, Kromhout H, Kauppinen T, et al. Statistical modelling of the determinants of historical exposure to bitumen and polycyclic aromatic hydrocarbons among paving workers. Ann Occup Hyg. 2000;44:43–56.
- Creely KS, Cowie H, Van Tongeren M, et al. Trends in inhalation exposure-a review of the data in the published scientific literature. Ann Occup Hyg. 2007;51:665–678.
- Andersen A, Barlow L, Engeland A, et al. Work-related cancer in the Nordic countries. Scand J Work Environ Health. 1999;25 (Suppl 2): 1–116.
- Pukkala E, Martinsen JI, Lynge E, et al. Occupation and cancer - follow-up of 15 million people in five Nordic countries. Acta Oncol. 2009;48:646–790.
- Axelson O, Steenland K. Indirect methods of assessing the effects of tobacco use in occupational studies. Am J Ind Med. 1988;13:105–118.
- Haldorsen T, Andersen A, Boffetta P. Smoking-adjusted incidence of lung cancer by occupation among Norwegian men. Cancer Causes Control. 2004;15:139–147.
- Haldorsen T, Martinsen JI, Kjærheim K, et al. Adjustment for tobacco smoking and alcohol consumption by simultaneous analysis of several types of cancer. Cancer Causes Control. 2017;28:155–165.
- Blair A, Stewart P, Lubin JH, et al. Methodological issues regarding confounding and exposure misclassification in epidemiological studies of occupational exposures. Am J Ind Med. 2007;50:199–207.
- Carstensen JM, Pershagen G, Eklund G. Smoking-adjusted incidence of lung cancer among Swedish men in different occupations. Int J Epidemiol. 1988;17:753–758.
- IARC monographs on the evaluation of carcinogenic risks to humans. Tobacco smoking. IARC Monogr Eval Carcinog Risk Hum 1986;38:1–114.
- Pukkala E, Engholm G, Hojsgaard Schmidt LK, et al. Nordic Cancer Registries – an overview of their procedures and data comparability. Acta Oncol. 2017;11:1–16.
- Armitage P. Statistical methods in medical research. Oxford, Blackwell; 1971.
- Al-Zalabani AH, Stewart KF, Wesselius A, et al. Modifiable risk factors for the prevention of bladder cancer: a systematic review of meta-analyses. Eur J Epidemiol. 2016;31:811–851.
- Cumberbatch MG, Cox A, Teare D, et al. Contemporary Occupational Carcinogen Exposure and Bladder Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2015;1:1282–1290.
- IARC monographs on the evaluation of carcinogenic risks to humans. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr Eval Carcinog Risk Hum. 2010;92:89–93. [Accessed 2017 April 18]. Available from: http://monographs.iarc.fr/ENG/Monographs/vol92/mono92.pdf.
- Boffetta P, Jourenkova N, Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control. 1997;8:444–472.
- Hogstedt C, Jansson C, Hugosson M, et al. Cancer incidence in a cohort of Swedish chimney sweeps, 1958-2006. Am J Public Health. 2013;103:1708–1714.
- Cassidy A, Wang W, Wu X, et al. Risk of urinary bladder cancer: a case-control analysis of industry and occupation. BMC Cancer. 2009;9:443.
- Porru S, Aulenti V, Donato F, et al. Bladder cancer and occupation: a case-control study in northern Italy. Occup Environ Med. 1996;53:6–10.
- Ji J, Granstrom C, Hemminki K. Occupation and bladder cancer: a cohort study in Sweden. Br J Cancer. 2005;92:1276–1278.
- Reijula J, Kjaerheim K, Lynge E, et al. Cancer incidence among waiters: 45 years of follow-up in five Nordic countries. Scand J Public Health. 2015;43:204–211.
- Harling M, Schablon A, Schedlbauer G, et al. Bladder cancer among hairdressers: a meta-analysis. Occup Environ Med. 2010;67:351–358.
- Takkouche B, Regueira MC, Montes MA. Risk of cancer among hairdressers and related workers: a meta-analysis. Int J Epidemiol. 2009;38:1512–1531.
- IARC monographs on the evaluation of carcinogenic risks to humans. Occupational exposure of hairdressers and barbers and personal use of hair colorants; some hair dyes; cosmetic colorants, industrial dyestuffs and aromatic amines. IARC Monogr Eval Carcinog Risk Hum. 1993;57:1–437. [cited 2016 Jun 28]. Available from: http://monographs.iarc.fr/ENG/Monographs/vol57/mono57.pdf.
- Ferris J, Garcia J, Berbel O, et al. Constitutional and occupational risk factors associated with bladder cancer. Actas Urol Esp. 2013;37:513–522.
- Pukkala E, Nokso-Koivisto P, Roponen P. Changing cancer risk pattern among Finnish hairdressers. Int Arch Occup Environ Health. 1992;64:39–42.
- Bolt HM, Golka K. The debate on carcinogenicity of permanent hair dyes: new insights. Crit Rev Toxicol. 2007;37:521–536.
- Hadkhale K, Martinsen JI, Weiderpass E, et al. Occupation and risk of bladder cancer in nordic countries. J Occup Environ Med. 2016;58:e301–e307.
- IARC monographs on the evaluation of carcinogenic risks to humans. Printing processes and printing inks, carbon black and some nitro compounds. IARC Monogr Eval Carcinog Risk Hum 1996;65:1–115. [cited 2017 March 28]. Available from: http://monographs.iarc.fr/ENG/Monographs/vol65/mono65-5.pdf.
- International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risk to humans. Agents classified by the IARC monographs. 2017; Vol 1 to 118. [cited 2017 Apr 21]. Available from: http://monographs.iarc.fr/ENG/Classification/latest_classif.php.
- Kriebel D, Zeka A, Eisen EA, et al. Quantitative evaluation of the effects of uncontrolled confounding by alcohol and tobacco in occupational cancer studies. Int J Epidemiol. 2004;33:1040–1045.
- Helakorpi S, Patja K, Prattala R, et al. Health behaviour and health among the Finnish adult population. Helsinki: National Public Health Institute; 2003.
- Ronneberg A, Lund KE, Hafstad A. Lifetime smoking habits among Norwegian men and women born between 1890 and 1974. Int J Epidemiol. 1994;23:267–276.
- Notkola V, Pajunen A, Leino-Arjas P. Occupational mortality by cause in Finland 1971–1991 and occupational mobility. SVT health, Vol 1. Helsinki (Finland): Statistics Finland; 1997.
- Pukkala E, Guo J, Kyyronen P, et al. National job-exposure matrix in analyses of census-based estimates of occupational cancer risk. Scand J Work Environ Health. 2005;31:97–107.
- Pukkala E, Teppo L, Hakulinen T, et al. Occupation and smoking as risk determinants of lung cancer. Int J Epidemiol. 1983;12:290–296.
- Randem BG, Burstyn I, Langard S, et al. Cancer incidence of Nordic asphalt workers. Scand J Work Environ Health. 2004;30:350–355.
- Shen H, Huang Y, Wang R, et al. Global atmospheric emissions of polycyclic aromatic hydrocarbons from 1960 to 2008 and future predictions. Environ Sci Technol. 2013;47:6415–6424.
- Takala J. Eliminating Occupational Cancer in Europe and Globally. European Trade Union Institute (ETUI) Research Paper Series. ETUI Working Paper. 2015 [cited 2018 Apr 6]. Available from: https://www.etui.org/Publications2/Working-Papers/Eliminating-occupational-cancer-in-Europe-and-globally.
