Abstract
Background: Stereotactic body radiotherapy (SBRT) for pulmonary oligometastatic disease achieves excellent treatment outcomes in terms of local control and toxicity. Patients treated with SBRT are often elderly and have multiple co-morbidities. This subset of patients may experience different survival as compared to young and fit patients subjected to radical metastasectomies. The purpose of this retrospective study was to evaluate OS and identify factors associated with OS for inoperable pulmonary oligometastases treated with SBRT.
Material and methods: Criteria used for selection of patients with oligometastases included: metastases limited to ≤2 organs and in total ≤5 metastases at the time of treatment. Peripheral tumors were treated with 51 Gy to 60 Gy in three fractions or a single fraction of 30 Gy. Central tumors received a dose of 45–60 Gy in 5–8 fractions. Survival probabilities were estimated by means of Kaplan–Meier method and the relation between potential prognostic factors and OS was studied by means of Cox regression analyses.
Results: In this study, 327 inoperable pulmonary oligometastases in 206 patients were treated with SBRT from the year 2005 to 2015. Primary sites of pulmonary oligometastases included colorectal carcinoma (n = 118), lung carcinoma (n = 36), melanoma (n = 11), sarcoma (n = 10), breast carcinoma (n = 7), and other tumors sites (n = 24). Median follow-up was 26 months. Median survival was 33 months. The 2-year and 5-year OS rates were 63% and 30%, respectively. On univariate analysis synchronous oligometastases (HR 0.59) and colorectal primary (HR 0.64) were associated with improved OS. On multivariable analysis synchronous oligometastases (HR 0.56), colorectal primary (HR 0.62) and tumor size <3 cm (HR 0.68) were independently associated with OS.
Conclusions: SBRT to pulmonary oligometastases was associated with a 2-year OS of 63%. Tumor size <3 cm and colorectal primary tumors experienced improved OS compared to tumors >3 cm and non-colorectal primary tumors.
Introduction
The oligometastatic disease represents a potentially curable state of distant metastases. It refers to a state which is ‘intermediate between purely localized lesions and widely metastatic disease’. The oligometastatic state was hypothesized in 1995 [Citation1], although efforts to eradicate metastases by local therapies have been documented as early in the 1930s [Citation2]. Existence of oligometastases has been confirmed for various cancer sites and use of aggressive local treatment of oligometastases has translated into improvement of overall survival (OS) [Citation2,Citation3]. For example, the long-term results of a randomized phase II study of the EORTC-NCRI CCSG-ALM Intergroup reported improved OS after Radiofrequency Ablation (RFA) with chemotherapy of unresectable colorectal liver metastases compared to chemotherapy alone [Citation4]. Furthermore, recently, a randomized phase II trial also reported improved progression-free survival (PFS) if local consolidative therapy was added to systemic therapy for patients with oligometastatic non-small cell lung cancer (NSCLC) [Citation5].
Lung is the most commonly involved organ by distant metastases. Pulmonary metastasectomy is a time-tested treatment approach for patients with oligometastatic lung disease [Citation2,Citation3,Citation6–8]. Complete surgical excision of pulmonary oligometastases results in OS of 26% at 10 years and 22% at 15 years [Citation3]. Not all patients are suitable for radical excision; patients can be inoperable due to central location of lesions, presence of one or more lesions in both lungs, history of lobectomy or pneumonectomy, or patients may be poor surgical candidates due to other medical conditions. Other treatment options for patients diagnosed with pulmonary oligometastases include stereotactic body radiotherapy (SBRT), RFA, laser therapy, and chemotherapy. SBRT is a modern radiation technique that delivers a high dose to the tumor in a small number of fractions. For patients with pulmonary oligometastatic disease, SBRT achieves excellent local control and is associated with limited toxicity [Citation9–21]. Patients treated with SBRT are usually not suitable for surgery, are often elderly and have multiple co-morbidities. Given the limited lifespan, this subset of patients may experience different survival as compared to young and fit patients subjected to radical metastasectomies. Therefore, we conducted the present study with the aim to evaluate OS and identify prognostic factors associated with OS for inoperable pulmonary oligometastases treated with SBRT.
Material and methods
Patient selection
This retrospective study was approved by the institutional medical ethics committee of the Erasmus Medical Center (EMC) with approval number MEC-2016-729. Criteria used for selection of patients with oligometastatic disease included: metastases limited to 2 organs and in total 5 metastases at the time of treatment. Patients were considered inoperable in presence of severe comorbidity or when tumors were unresectable. Patients previously treated for oligometastases with surgery or chemotherapy but not with SBRT who developed oligo-recurrence were also eligible. Exclusion criteria were patients with polymetastatic progression or patients with prior thoracic radiotherapy. From 2005 to 2015, 327 inoperable pulmonary metastases in 206 patients were treated with SBRT. Histopathological confirmation of metastases was considered in patients whenever safe and feasible and was performed in 42% of the patients. All inoperable pulmonary metastases were treated with SBRT and all extra-pulmonary oligometastases were treated with surgery, SBRT or RFA, depending on the decision of the multidisciplinary tumor board.
Treatment schedules
Details of SBRT procedure and dose fractionation schedules used for pulmonary oligometastases at the institution have been previously described in detail [Citation21–24]. Briefly, the radiation schedule used did depend on the location of tumor. Peripheral lung metastases were treated to 51 Gy or 60 Gy in 3 fractions or to 30 Gy in a single fraction, whereas central tumors (located within 2 cm of trachea and main bronchus) received 50–60 Gy in 5 fractions, except when the tumor was located close to esophagus. In that case, metastases were treated with 6–7 fractions of 8 Gy or 7 fractions of 7 Gy. Dose to target volume was prescribed at the 70–90% isodose line (median 78%), covering at least 95% of the Planning Target Volume. Similar biological effect for different dose-fractionation schedules used in our study was assessed by converting the physical dose to the biological equivalent dose (BED10), assuming an α/β ratio of 10 Gy. To account for the dose calculation inaccuracy of the Ray Tracing as compared to Monte Carlo algorithm [Citation25,Citation26], the BED10 calculated with the Ray Tracing algorithm was corrected by reducing it by 12% for central tumors and 17% to 21% for peripheral tumors [Citation25].
Clinical follow-up was performed at 3 weeks after SBRT and at 3, 6, 12, 18, and 24 months after treatment and yearly thereafter. All acute and late adverse events were reported and scored for severity using NCI Common Terminology Criteria for Adverse Events, version 3.0. Only the highest score of acute and late toxicity of each patient was used to determine the toxicity of the whole group.
Definition of endpoints
OS was calculated from the date of first SBRT session to date of death or date of last follow up for alive patients. Progression-free survival (PFS) was calculated from the date of first SBRT to date of local, regional or distant relapse whichever happened first. Patients without death or any relapse on last follow up were censored. Time to local recurrence was calculated from start date of SBRT to date of local recurrence. Patients who did not experience local recurrence were censored at the date of the last follow-up. Toxicities were recorded as per NCI Common Terminology Criteria for Adverse Events, version 3.0. Only the highest score of acute and late toxicities of each patient was used to calculate the toxicity.
Factors evaluated for prognosis
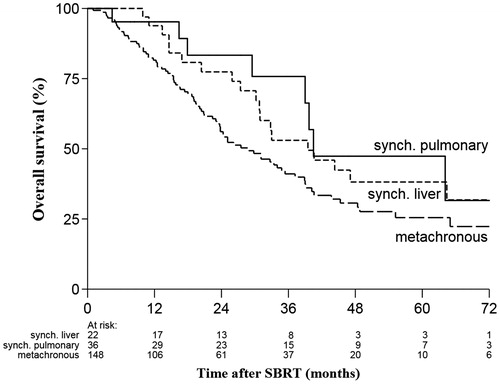
Variables included as prognostic factors were: age, gender, primary site, metachronous versus synchronous tumors, number of metastases in body, number of metastases in lungs, presence of extra-pulmonary metastases, delivery of pre-SBRT chemotherapy, BED10, fractionation schedule of SBRT, size of largest pulmonary metastases, delay in initiation of SBRT for ≥4 months, presence of comorbidities, location of lung metastases (central versus peripheral) and location of metastases in lower lobes versus other locations. Comorbidity was evaluated with the Charlson Comorbidity Index (CCI) and the Cumulative Illness Rating Scale (CIRS). Synchronous metastases were defined as presence of metastases within 5 months of diagnosis of the primary tumor whereas rest was labeled as metachronous tumors. Synchronous oligometastases either directly involved the lungs or initially involved liver and then later developed oligo-recurrence to the lung and/or other organs. To account for this variation, pulmonary oligometastases were divided into three categories: (1) synchronous lung oligometastases (n = 36); (2) synchronous liver oligometastases followed by the development of oligometastases to the lungs (n = 22); and (3) metachronous oligometastases (n = 148).
Statistical considerations
Survival estimates were calculated using the Kaplan–Meier method. Cox regression was used to obtain the Hazard Ratio (HR) and 95% confidence interval (CI) for various covariates. Covariates with a p-value ≤.20 were considered for multivariable analyses (MVA), which was also carried out by a Cox proportional hazards model. The MVA was carried out using backward selection where a threshold of p < .05 was used. All analyses were performed using IBM SPSS statistics version 22.0 software package (SPSS Inc., Chicago, IL, USA) and Stata version 14.0 (StataCorp. 2015. Stata: Release 14. Statistical Software, College Station, TX: StataCorp LP).
Results
Patient and tumor characteristics
In 206 patients, 327 pulmonary oligometastases were treated with SBRT between 2005 and 2015. Primary sites of pulmonary oligometastases included colorectal carcinoma (n = 118), lung carcinoma (n = 36), melanoma (n = 11), sarcoma (n = 10), breast carcinoma (n = 7), and other tumors sites (n = 24). Majority of patients (n = 148) had metachronous tumors. One-hundred-fifty-four patients had more than one metastasis and extra-pulmonary metastases were present in 86 patients. Ninety-nine patients received chemotherapy before start of the SBRT. Patient and tumor characteristics are shown in .
Table 1. Patient characteristics (N = 206).
Survival and local control
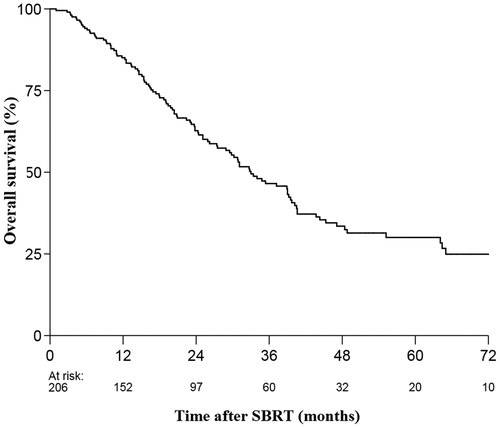
At the time of analysis 112 patients (54%) had died. Median follow-up for alive patients was 26 months (range: 2–102 months). Median OS in the entire cohort was 33 months. The 2-, 3-, and 5-year OS rates were 63%, 47% and 30%, respectively (). Median progression-free survival (PFS) was 13 months. The 2-, 3-, and 5-year PFS rates were 36%, 25%, and 16%, respectively. Local control at 2-, 3-, and 5-year was 85%, 83%, and 81%, respectively.
Prognostic factors
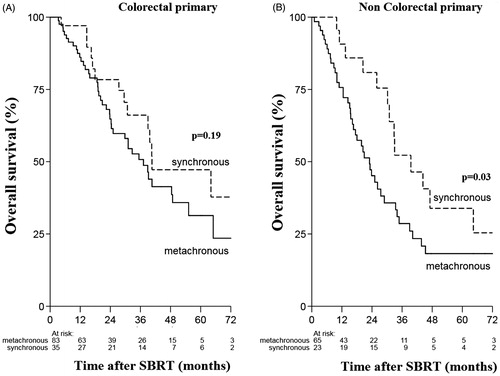
The univariate analyses showed that synchronous metastases (HR 0.59, 95% CI 0.38–0.92, p = .019) and colorectal primary site (HR 0.64, 95% CI 0.44–0.92; p = .018) were significantly associated with better OS (, ). In the multivariable analysis, synchronous metastases (HR 0.56, 95% CI 0.36–0.86; p = .009), colorectal primary site (HR 0.62, 95% CI 0.42–0.89, p = .011) and tumor size <3 cm (HR 0.68, 95% CI 0.46–0.99, p = .046) remained as independent factors significantly associated with better OS, whereas the variables age, pre-SBRT chemotherapy and BED dropped out of the model ().
Figure 2. (A) Overall survival for synchronous and metachronous oligometastases (B) Overall survival for colorectal and non colorectal primary tumors.
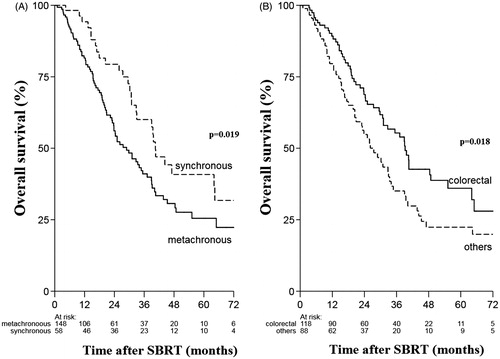
Table 2. Prognostic factors univariate analysis.
Table3. Prognostic factors on multivariable analysis.
Patients with synchronous liver metastases (n = 22) that later developed pulmonary oligo-recurrence had a median OS of 40.5 months, synchronous pulmonary oligometastases (n = 36) had median OS of 39.5 months and median OS for metachronous pulmonary oligometastases (n = 148) was 29 months. However, these results were not significant (). On subset analysis, patients with synchronous metastases from noncolorectal primaries experienced significantly better OS than metachronous tumors (p = .026) ().
SBRT related toxicity
SBRT was tolerated very well and there was no treatment-related death. SBRT toxicity was scored retrospectively. Acute grade 2 toxicities were experienced by <5% of the patients. Acute grade 3 toxicities experienced included grade 3 dyspnea (n = 3), chest pain (n = 1) and both dyspnea and fatigue (n = 1). As regards to late toxicity, 7.5% of the patients experienced late grade 2 cough and 6% experienced grade 2 fatigue.
Discussion
In this cohort 2-year and 5-year OS rates were 63% and 30%, respectively. The study demonstrates that SBRT achieves favorable long-term survival in patients ineligible for metastasectomy. Survival achieved in our cohort of patients is particularly encouraging as the majority of patients had multiple comorbidities; more than 42% had extra-pulmonary metastases, 75% had more than one metastases, and more than half of the patients were not treated with chemotherapy before the start of SBRT. The largest multi-institutional study that treated patients of pulmonary oligometastases with SBRT reported 2-year and 5-year OS of 53% and 24%, respectively [Citation27]. In this study, 5-year OS was 24% in the DEGRO cohort and reached 31% and 38% in the Aarhus and Torino cohorts, respectively [Citation27]. Although treating a different subset of patients with oligometastatic disease, our results are comparable with the surgical series [Citation3,Citation7]. In the meta-analysis conducted by the International Registry of Lung Metastases [Citation3], 5-year OS was 36% for completely resected pulmonary oligometastases and 5-year OS for incompletely resected tumors was 13%.
Interestingly, unlike other studies [Citation12,Citation16,Citation20,Citation27] this study identified synchronous metastases as an independent prognostic factor associated with better OS. One of the earliest studies that treated 321 patients with oligometastases with SBRT reported that metachronous tumors were independently associated with better OS (HR 0.71; p = .02) as compared to synchronous tumors [Citation12]. Similarly, in another large cohort of oligometastatic patients treated with SBRT, synchronous tumors were independently associated with worse OS (HR1.5, p = .007) than metachronous oligometastases [Citation28]. However, in the present study patients with synchronous metastases had 44% lower risk of dying than patients with metachronous metastases (HR 0.56 95% CI 0.36–0.86). Given the retrospective nature of the study, our findings are prone to selection bias. Selection bias could have resulted due to the fact that synchronous metastases that develop rapid disease progression were excluded in the present study and only synchronous tumors with indolent biology were included. However, we cannot rule out similar selection bias for metachronous tumors. A recently published study showed that radical treatment for oligometastatic lung cancer was equally effective for synchronous and metachronous tumors [Citation29]. A meta-analysis that evaluated 1800 patients diagnosed with multiple primary lung cancers concluded that despite the tumor-free interval between the first and second tumor in metachronous tumors, there was no significant difference in survival between synchronous and metachronous tumors [Citation30]. Taken together with our results, these findings may reflect existing biological heterogeneity of metastatic spread. MicroRNA expression has been evaluated as an initial step to demonstrate molecular basis for oligometastatic spread. After treatment with aggressive metastases directed therapies, microRNA signatures have been shown to correlate with propensity to develop polymetastatic vs. oligometastatic disease [Citation31,Citation32]. In future, tumor biology will play an integral part in selecting patients with oligometastases that are likely to benefit from aggressive therapies. This is an area of active research and in future has potential to provide insight into disease progression of synchronous and metachronous oligometastatic disease.
In this study, colorectal cancers were associated with better OS as compared to other tumor sites. Patients with colorectal oligometastases had 38% lower risk of dying than patients with other primary tumor sites (HR 0.62, 95% CI 0.42–0.89). The DEGRO group, having the largest cohort of patients with pulmonary oligometastases treated with SBRT, has also confirmed the impact of primary tumor site on OS [Citation27]. In this multi-institutional study, renal cell carcinoma showed the most favorable OS followed by colorectal primary cancers, while tumors from other sites such as esophagus and melanoma showed poorer OS. The favorable OS for colorectal primary tumors may be attributed to the use of aggressive local therapies for primary site and oligometastases, and availability of effective as well as multiple lines of chemotherapy [Citation3,Citation6–8,Citation12]. Few studies have reported worse tumor control for colorectal histology as compared to non colorectal histology [Citation33,Citation34]. The multi-intuitional Japanese study showed that 3-year local progression-free rate of patients with colorectal origin was significantly worse (39%) than that of non-colorectal origin (82%). The MVA also revealed that primary CRC was the only significant prognostic factor for LC. However, there was no significant difference in OS between CRC and other histologies [Citation33]. Similarly, Takeda et al reported 2-year LC rate of 80% for colorectal pulmonary oligometastases as compared to 94% for non colorectal histology. MVA in this study revealed that only tumor origin was significant associated with wore LC, unfortunately, this study did not evaluate OS. The authors concluded that LC for pulmonary oligometastases from CRC was worse than that from other origins and suggested dose escalation to achieve better LC [Citation34]. Indeed this holds true, in a recently published study where majority of pulmonary metastases were treated with BED10 >100 Gy reported no significant difference in LC for CRC versus other non CRC histology [Citation21].
In the present study tumor size <3 cm was significantly associated with better OS. Maximum diameter of tumor <3 cm was associated 32% lower risk of dying as compared to tumors >3 cm (HR 0.68, 95% CI 0.46–0.99). Similar findings have been reported in the literature [Citation27,Citation31].
Extra-pulmonary metastases and number of metastases were not significantly associated with OS. This result is comparable to a study evaluating 309 oligometastatic patients with different locations of their metastases that also concluded that the present number of oligometastases did not have an impact on OS [Citation28]. Results from one of the earliest studies that treated 587 oligometastases in 321 patients with SBRT, revealed that delivery of pre-SBRT chemotherapy was independently associated with favorable OS [Citation12]. However, in the current study as well as in the study by the DEGRO group this relation between pre-SBRT chemo and OS could not be established [Citation27].
Median PFS in our cohort was 13 months. Fifty percent of patients experienced disease progression within a year and by the third year 75% had disease progression. Limitation of the study includes its retrospective nature, was conducted in a single center and is prone to selection bias. We did not include disease-free interval as prognostic factor, SBRT toxicity was scored retrospectively. Other limitations are that site of extra-pulmonary organs involved was not collected, nor their treatment by local therapies and use of multiple lines of chemotherapy used were not taken into account in our analyses.
SBRT for inoperable pulmonary oligometastases was associated with OS of 63% at 2 years and 30% at 5-years. Our study is the first study one to report that synchronous oligometastases are independently associated with better OS than metachronous oligometastases. However, our results need to be further validated by other studies. Molecular markers may in future help to identify the course of disease progression for oligometastases. Other factors independently associated with better OS were colorectal primary and tumor size. After an external validation of our results, all these factors may be used to refine SBRT practice for pulmonary oligometastases.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10.
- Dongen JA, Slooten EA. The surgical treatment of pulmonary metastases. Cancer Treatment Rev. 1978;5:29–48.
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113:37–49.
- Ruers T, Punt CJA, van Coevorden F, et al. Radiofrequency ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRC LM): long-term survival results of a randomized phase II study of the EORTC-NCRI CCSG-ALM Intergroup 40004 (CLOCC). J Clin Oncol. 2015;33:3501.
- Gomez DR, Blumenschein GR, Jr Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17:1672–1682.
- Simmonds PC, Primrose JN, Colquitt JL, et al. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982–999.
- Casiraghi M, De Pas T, Maisonneuve P, et al. A 10-year single-center experience on 708 lung metastasectomies: the evidence of the international registry of lung metastases. J Thorac Oncol. 2011;6:1373–1378.
- Tanvetyanon T, Robinson LA, Schell MJ, et al. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small-cell lung cancer: a systematic review and pooled analysis. Jco. 2008;26:1142–1147.
- Siva S, MacManus M, Ball D. Stereotactic radiotherapy for pulmonary oligometastases a systematic review. J Thorac Oncol. 2010;5:1091–1099.
- Nuyttens JJ, Voort van Zyp CMG, Verhoef C, et al. Stereotactic body radiation therapy for oligometastases to the lung: a phase 2 study. Int J Radiat Oncol Biol Phys. 2015;91:337–343.
- Hof H, Hoess A, Oetzel D, et al. Stereotactic single-dose radiotherapy of lung metastases. Strahlenther Onkol. 2007;183:673–678.
- Fode MM, Høyer M. Survival and prognostic factors in 321 patients treated with stereotactic body radiotherapy for oligo-metastases. Radiother Oncol. 2015;114:155–160.
- Norihisa Y, Nagata Y, Takayama K, et al. Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys. 2008;72:398–403.
- Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. Jco. 2009;27:1579–1584.
- Zhang Y, Xiao JP, Zhang HZ, et al. Stereotactic body radiation therapy favors long-term overall survival in patients with lung metastases: five-year experience of a single-institution. Chin Med J. 2011;124:4132–4137.
- Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for lung metastases. Lung Cancer. 2012;75:77–81.
- Oh D, Ahn YC, Seo JM, et al. Potentially curative stereotactic body radiation therapy (SBRT) for single or oligometastasis to the lung. Acta Oncol. 2012;51:596–602.
- Navarria P, Ascolese M, Tomatis S, et al. Stereotactic body radiotherapy (sbrt). in lung oligometastatic patients: role of local treatments. Radiat Oncol. 2014;9:91.
- Aoki M, Hatayama Y, Kawaguchi H, et al. Stereotactic body radiotherapy for lung metastases as oligo-recurrence: a single institutional study. J Radiat Res. 2016;57:55–61.
- Rieber J, Streblow J, Uhlmann L, et al. Stereotactic body radiotherapy (SBRT) for medically inoperable lung metastases—a pooled analysis of the German working group “stereotactic radiotherapy”. Lung Cancer.2016;97:51–58.
- Sharma A, Duijm M, Oomen-de H, et al. Factors affecting local control for pulmonary oligometastasis treated with stereotactic body radiotherapy. Acta Oncol. 2018;57:1031–1037.
- Van der Voort Van Zyp NC, Prévost JB, Hoogeman MS, et al. Stereotactic radiotherapy with real-time tumor tracking for non-small cell lung cancer: clinical outcome. Radiother Oncol. 2009;91:296–300.
- Nuyttens JJ, van de Pol M. The CyberKnife radiosurgery system for lung cancer. Expert Rev Med Devices. 2012;9:465–475.
- Hoogeman M, Prevost JB, Nuyttens J, et al. Clinical accuracy of the respiratory tumor tracking system of the cyberknife: assessment by analysis of log files. Int J Radiat Oncol Biol Phys. 2009;74:297–303.
- van der Voort van Zyp NC, Hoogeman MS, van de Water S, et al. Clinical introduction of Monte Carlo treatment planning: a different prescription dose for non-small cell lung cancer according to tumor location and size. Radiother Oncol. 2010;96:55–60.
- Wilcox EE, Daskalov GM, Lincoln H, et al. Comparison of planned dose distributions calculated by Monte Carlo and Ray-Tracing algorithms for the treatment of lung tumors with cyberknife: a preliminary study in 33 patients. Int J Radiat Oncol Biol Phys. 2010; 77:277–284.
- Tanadini-Lang S, Rieber J, Filippi AR, et al. Nomogram based overall survival prediction in stereotactic body radiotherapy for oligo-metastatic lung disease. Radiother Oncol. 2017;123:182–188.
- De Vin T, Engels B, Gevaert T, et al. Stereotactic radiotherapy for oligometastatic cancer: a prognostic model for survival. Ann Oncol. 2014;25:467–471.
- Fleckenstein J, Petroff A, Schäfers H-J, et al. Long-term outcomes in radically treated synchronous vs. metachronous oligometastatic non-small-cell lung cancer. BMC Cancer. 2016;16:348
- Jiang L, He J, Shi X, et al. Prognosis of synchronous and metachronous multiple primary lung cancers: systematic review and meta-analysis. Lung Cancer. 2015;87:303–310.
- Lussier YA, Xing HR, Salama JK, et al. MicroRNA expression characterizes oligometastasis(es). PLoS One. 2011;6:e28650
- Lussier YA, Khodarev NN, Regan K, et al. Oligo- and polymetastatic progression in lung metastasis(es) patients is associated with specific microRNAs. PLoS One. 2012;7:e50141
- Onishi H, Matsumoto Y, Miyakawa A, et al. Japanese multiinstitutional study of stereotactic body radiation therapy for totally 380 patients with lung metastases. Int J Radiat Oncol Biol Phys. 2014;90:S46–S47.
- Takeda A, Kunieda E, Ohashi T, et al. Stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer and other primary cancers in comparison with primary lung cancer. Radiother Oncol. 2011;101:255–259.