Abstract
Background: Gonadal dysfunction is one of the major late complications after cancer diagnosis and treatment. The best markers of ovarian reserve in clinical practice are antral follicle count (AFC) and ovarian volume. We aimed to study the prevalence of premature ovarian insufficiency (POI) and evaluate anti-Müllerian hormone (AMH) and other serum markers for ovarian function in adult women who were childhood cancer survivors (CCS) in comparison with a control group.
Material and methods: Altogether, 167 female CCS were compared to 164 matched controls. Prevalence of POI was documented and serum levels of AMH, inhibin B, follicle stimulating hormone (FSH), and estradiol (E2) were compared with AFC and ovarian volume.
Results: POI was reported in 22 (13%) of the CCS and in none of the controls. Serum levels of AMH, inhibin B, and FSH, but not E2, correlated significantly with AFC and ovarian volume; AMH showed the highest correlation. There was no difference between CCS and controls regarding the different serum markers as measured by linear regression analysis. ROC curve AUC for primary POI showed the highest values for AMH (0.930) and AFC (0.944). For AFC <10, ROC curve AUC showed highest value for AMH for CCS (0.866) and controls (0.878). In a subgroup of female CCS <40 years (n = 120), the results were similar.
Conclusion: We found POI in 13% among CCS, slightly more than in other studies. Serum levels of AMH, inhibin B, and FSH correlated significantly with AFC and ovarian volume, and no difference was noted between CCS and controls. AMH was the most reliable serum marker for ovarian function in terms of POI and low AFC.
Introduction
Cancer treatment during childhood is known to increase the risk of damage to the ovaries with subsequent loss of follicular reserve. Studies have shown that alkylating agents and direct irradiation are the most toxic treatments to the ovaries [Citation1,Citation2] although the exact toxic doses or combinations of chemotherapeutic drugs remain undefined. A potential side effect in female cancer survivors is accelerated follicle loss with impaired ovarian function appearing either as absent puberty or premature menopause [Citation3]. The term premature ovarian insufficiency (POI) is a clinical diagnosis defined as loss of ovarian function before age of 40 years. It is characterized by menstrual disturbances, amenorrhea or oligomenorrhea with raised FSH and low estradiol [Citation4,Citation5]. Estrogen deficiency in women increases the risk of osteoporosis, cardiovascular diseases, and metabolic syndrome and is associated with impaired reproductive capacity [Citation4,Citation6]. It is therefore, crucial to identify the women at risk of developing POI to manage further complications.
Several methods are used in clinical practice to assess ovarian reserve: antral follicle count (AFC), ovarian volume, serum levels of follicle stimulating hormone (FSH), estradiol and inhibin B [Citation7]. FSH, estradiol and inhibin B are all part of the feedback system, which makes their serum levels dependent on each other [Citation8]. The use of oral contraceptives (OC) and hormone replacement therapy (HRT) aggravate interpretation of FSH and sex steroids levels regarding ovarian status. In addition, the ovarian function needs to be significantly decreased for FSH to be elevated [Citation8], which is a disadvantage when predicting likelihood and timing of premature menopause. Because AFC (the sum of the growing follicles 2–10 mm in diameters in the ovaries) appears to correlate well with the primordial follicle pool, this method is currently the most accurate way to predict ovarian reserve [Citation9]. However, AFC requires a transvaginal ultrasound, an examination that young females may experience as discomfort.
Although AMH has been identified as a marker for ovarian reserve, it is not used in follow-up programs for female childhood cancer survivors (CCS) [Citation3]. In females, AMH is detectable at birth and then increases throughout childhood and puberty until it peaks at the age of 25 years [Citation10]. Thereafter, the AMH level declines steadily until menopause when it reaches undetectable levels [Citation11]. To date, AMH is mostly used to predict response to in vitro fertilization therapy [Citation12]. However, recent studies show a decline in AMH levels in CCS when compared to controls [Citation13–19]. This difference indicates that AMH levels could serve a serum marker to screen for impaired ovarian reserve. AMH correlates well with AFC in healthy women [Citation20], but no studies have established whether AMH is correspondingly correlated to AFC in female CCS [Citation3]. This study examines the prevalence of POI and also the value of AMH, inhibin B, FSH and E2 as markers for POI and low AFC/impaired ovarian function in female adult CCS compared with matched controls.
Subject and methods
Participants
Eligible participants were recruited from the Swedish Cancer Registry. The inclusion criteria were females treated for childhood cancer in southern Sweden between 1964 and 2008. In addition, these patients were below 18 years of age at diagnosis and had completed treatment more than two years before inclusion. The exclusion criteria were females with rare tumors or with focal tumors outside of the central nervous system treated with surgery only. Each patient was matched with a control regarding sex, date of birth, ethnicity and smoking habits through the Swedish Population Registry.
POI was diagnosed in those with a history of primary or secondary amenorrhea below age 40 with subsequent estrogen replacement therapy in combination with very low AMH (<0.1 ng/ml or undetectable AMH <0.023 ng/ml). Because almost all women with ovarian insufficiency took HRT at the time of examination, estradiol and FSH could not be used to define POI. Participants were excluded from the analyses of FSH and estradiol if they had ongoing OC as well as other hormonal birth control methods including progesterone as a pill, subcutaneous implant, or depot injection or HRT due to impact on the hypothalamic-pituitary-gonadal axis. However, participants with an intrauterine device (IUD) (both with progesterone and copper) were included, as it was shown that these devices do not affect the hormonal levels in females [Citation21].
The ovarian volumes were presented as the total volume of both ovaries. Only the women with estimated ovarian volumes on both sides were included. The same method was applied for AFC – the sum of follicles on both sides.
To determine whether the patient cohort was representative for the total group of female cancer survivors, data on offspring of the participants and nonparticipants were extracted from the Swedish Multi-Generation Registry, Statistics Sweden. From this registry, we obtained information about the number of children of each index person. For persons alive after 1990, this registry is almost complete with respect to parents and offspring. The distribution of women having 0, 1, 2, or 2+ children was 51, 14, 23 and 11% among study patients, 53, 13, 24 and 10% among those who declined participation, and 50, 26, 18 and 6% among those who did not respond to the study request, respectively.
Methods
All participants were examined at the Reproductive Medicine Center (RMC) at Skåne University Hospital Malmö, Sweden, between October 2010 and January 2015. The examination included questionnaires, blood samples for hormone levels, and physical examination including transvaginal ultrasound for measurement of the ovaries and AFC. We analyzed serum samples for AMH, inhibin B, FSH and 17β-estradiol (E2). Fasting blood samples were drawn during the early follicular phase (i.e., day 2–5 of the menstrual cycle) of the females with a regular menstrual cycle and on any day for the participants with amenorrhea as well as participants with OC or HRT. The blood samples were centrifuged and stored at –20 °C until further analysis.
Serum levels of AMH and inhibin B were measured at the Laboratory of Reproductive Biology, Copenhagen, Denmark. AMH levels were measured using specific ultrasensitive enzyme-linked immunosorbent assay (ELISA) kits with a detection limit of 0.023 ng/ml. All AMH values <0.023 ng/ml were stated as 0. Inhibin B was measured using MCA1312KZZ (Oxford Bio-Innovation Ltd., Oxfordshire, UK) with a detection limit of 20 pg/ml, and all values <20 pg/ml were stated as 0. The plasma levels of FSH (Roche) were measured at the Department of Clinical Chemistry, Skåne University Hospital, Malmö, Sweden. During the study an update in FSH methods occurred, 56 of the female childhood cancer survivors (CCS) had FSH values analyzed with a previous assay with a detection limit of 0.2 IU/L. All other CCS and all controls had FSH values analyzed with the most recent assay (Roche) with a detection limit of 0.1 IU/L. The lower detection levels and imprecision (coefficient of variation (CV) %) for the current and previous assay were presented in a study by Bobjer et al. [Citation22] in supplemental Figure 1C, Tables S1 and S2 in Appendix S1. The conversion factor is 0.893x−0.136, where x is the previous FSH value (R2 = 0.989). We analyzed the data with a transformation made for 56 CCS to the most recent FSH assay. FSH values <0.1 IU/L were stated as 0. Plasma levels of E2 (DELFIA PerkinElmer Inc.) were measured at the Department of Clinical Chemistry, Skåne University Hospital, Malmö, Sweden, with a detection limit of <10 pmol/L. After 1 July 2014, the method was improved with a detection limit of 3 pmol/L (LC-MSMS, NPU09357). All E2 values <10 pmol/L were stated as 0. Intra- and inter-assay coefficients of variation were <10% for both methods. Transvaginal ultrasound, performed with BK Medical Flex Focus 500 and BK Medical Pro Focus Scanner, was used to assess the volume of the ovaries as well as the antral follicle counts (follicles measuring 2–10 mm in diameter) on both sides. AFC <10 was used to define a diminished ovarian reserve [Citation23].
Data collected from the questionnaires included age at menarche, presence of primary or secondary amenorrhea, use of contraceptives or estrogen substitutes and number of pregnancies. Information about each patient’s diagnosis and treatment protocol, alkylating agents [Citation3], and irradiation was collected from the Childhood Cancer Registry and BORISS (Pediatric Oncology Registry in South Sweden). The distribution of diagnoses of childhood cancer was obtained from the Swedish Childhood Cancer Foundation [Citation24] to determine whether the cohort of study participants was representative for the entire population.
The study was approved by the Regional Ethics Committee, Medical Faculty, Lund University, Sweden (approval no. 523/2009). A written informed consent was obtained from all participants before study inclusion.
Statistical analysis
All statistical analyses were performed using IBM Statistical Package for the Social Science (SPSS) version 24. Descriptive data were expressed as count, percentage, and mean (minimum-maximum). Histograms were used to investigate whether the data were normally distributed. Continuous variables were analyzed using the independent sample t-test and nominal variables were calculated using the Fisher’s exact test. Correlation of the different variables was illustrated using scatter plots and further calculated with Pearson correlation. For somewhat skewed data (FSH) we added Mann-Whitney test and Spearman correlation. Linear regression was used to compare CCS and controls. Confidence intervals (CI) were set to 95%, and p values < .05 were considered statistically significant. ROC curve analyses were also used for evaluation of accuracy of the different test methods.
Results
Subjects
A total of 575 women were identified in the Swedish Cancer Registry that fulfilled the inclusion criteria. Of those, 244 women were excluded, mostly due to the presence of cell atypia and cancer in situ instead of mature cancer. In total, 331 women were eligible for the study. Representing 50% of eligible participants, 167 female CCS with a mean age of 34.3 (19.3–57.8) years were examined. Parameters of ovarian function were analyzed separately in those <40 years, representing a cohort of 120 CCS with a mean age of 30.0 (19.3–39.8) years.
Controls
Altogether, 167 control women were recruited. Three of the controls dropped out after examination, resulting in a total of 164 women with a mean age of 35.0 (19.3–58.0) years. Ovarian parameters were analyzed separately in women <40 years, resulting in 113 controls with a mean age of 30.3 (19.3–39.9) years.
Participant characteristics
Background characteristics are shown in . The distribution of childhood cancer diagnoses () was representative compared to the distribution of diagnoses in Sweden for females younger than 19 years [Citation24]. Altogether, 87 (52%) were treated with radiotherapy, 53 (32%) had cranial irradiation, 34 (20%) abdominal irradiation, 16 (10%) both cranial and abdominal irradiation, and 7 (4%) had total body irradiation (TBI). In addition, 126 (75%) were treated with chemotherapy and 81 (49%) with alkylating agents. Eleven (7%) had hematopoietic stem cell transplantation (HSCT) and in 19 (11%) only surgery was performed.
Table 1. Background characteristics for female childhood cancer survivors and controls.
Table 2. Distribution of diagnoses in the current study and for the female cancer population younger than 19 years old in Sweden between 1984 and 2010 [Citation24].
Analysis of serum markers of ovarian function compared to AFC and ovarian volume
Serum markers AMH, inhibin B, FSH and E2 were compared with AFC and ovarian volume. For analysis of AMH and inhibin B, there were 166 CCS and 163 controls. FSH levels were available in all CCS and controls, but in the final analyses only those without any hormonal treatment (HRT or OC) with the possible bias of serum levels were included, resulting in 102 CCS and 106 controls. E2 levels were available in all CCS and controls, but in two survivors and three controls, the analysis was performed with a non-sensitive method and therefore excluded. In the final data analyses, only those without any hormonal treatment with possible influence on the serum levels were included – 100 CCS and 105 controls.
Altogether, 135 CCS and 157 controls had an antral follicle cell count. In some patients, AFC could be documented although ovarian volume was not available, mainly because of difficulties to measure because of a dominant follicular cyst. In 129 CCS and 155 controls, ovarian volume was documented. Six different examiners performed the transvaginal ultrasound. Among those excluded were females with uni- or bilateral oophorectomy, non-visualized ovaries, ovarian cysts, or if they had not debuted sexually.
Premature ovarian insufficiency
POI was diagnosed in 22 CCS (13%). HRT as treatment was reported in 20 (12%) of all CCS, of which five due to hypothalamic/pituitary insufficiency. Of those with POI, one of 12 CCS <40 years at examination was not on any hormonal treatment. Of the seven CCS between 40 and 50 years at examination, four had stopped HRT and after age 50 years, one of three CCS used HRT. No participant in the control group reported either primary or secondary amenorrhea before age 40 years or the use of HRT.
AMH compared with AFC and ovarian volume
The AMH mean levels in the CCS group and the controls was 2.9 ng/ml and 3.1 ng/ml (p = .486), respectively. Subsequently, the AMH levels were compared with AFC and ovarian volume in the CCS and control group. The strongest correlation was noted between AMH and AFC in the CCS group and among controls. There were no significant differences between the groups ( and ).
Figure 1. Comparison of serum markers and AFC and ovarian volume for CCS and controls: CCS=●+filled line, controls=⋄+dashed line. (a) AMH–AFC; (b) AMH–ovarian volume; (c) Inhibin B–AFC; (d) Inhibin B–ovarian volume; (e) FSH–AFC; (f) FSH–ovarian volume. See for Pearson’s correlation results and comparison between the groups with linear regression analysis.
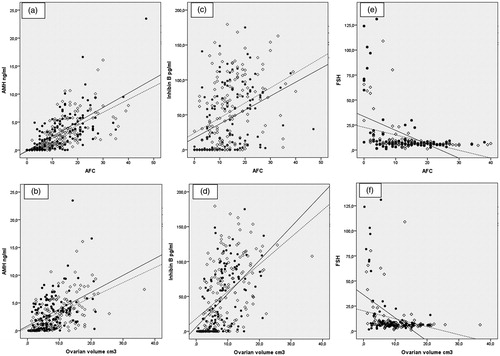
Table 3. Correlation between AMH, inhibin B, FSH, E2, AFC, and ovarian volume in the CCS and the control group and comparison between the groups.
Inhibin B compared with AFC and ovarian volume
The mean level of inhibin B was 37.7 pg/ml for the CCS and 53.2 pg/ml for the controls (p < .01). Inhibin B values correlated to AFC and ovarian volume in both CCS and controls. There were no significant differences between CCS and controls ( and ).
FSH compared with AFC and ovarian volume
Mean levels of FSH were 17.8 IU/L among CCS and 11.6 IU/L among controls (p = .049, t-test), and the median levels of FSH were 7.0 IU/L and 6.6 IU/L, respectively, among CCS and controls (p = .47, Mann-Whitney test). Levels of FSH showed a negative correlation to AFC and ovarian volume in both CCS and controls. No difference was noted between CCS and controls ( and ).
E2 compared to AFC and ovarian volume
The mean serum levels of 17β estradiol in the CCS group was 185.9 pmol/L and in controls 169.6 pmol/L (p = .48). E2 showed no correlation to either AFC or ovarian volume among both CCS and controls. Linear regression analysis showed no difference between the two groups () (Figure not shown).
AFC and ovarian volume
There was no difference in mean AFC in the group of CCS, 12.6 (0–47) and 14.2 (0–40) for the control group (p = .12). The mean ovarian volume was 7.7 cm3 (range 0.3–21.4) in the CCS group and 9.4 cm3 (range 0.9–36.7) in the control group (p = .01). Ovarian volume correlated to AFC in the CCS and controls (r = 0.528). Linear regression analysis showed no difference between the groups, although there was a tendency for smaller ovarian volume in the CCS compared to controls at a certain AFC (p = .051) () (Figure not shown).
ROC curve analysis for evaluation of accuracy of markers of ovarian function
We used the area under the ROC curve (AUC) to test how different markers could be used in the diagnosis of primary POI. We tested AMH, inhibin B, AFC and ovarian volume. FSH and E2 could not be evaluated as there were too few patients after excluding those who took HRT or OC. Test accuracy is shown for primary POI among the CCS and accuracy of diagnosing AFC <10 among both the CCS and controls (Supplementary Table 4 and ).
Figure 2. ROC curve analysis area under the curve (AUC). (a) Premature ovarian failure (POI) among childhood cancer survivors (CCS); (b) Antral follicle count (AFC) <10 among CCS; (c) AFC <10 among controls. See Supplementary Table 4 for specific values.

Results for women below age 40 years
We also analyzed ovarian parameters in the subjects younger than 40 years to evaluate if the inclusion of women of all ages influenced the results. When comparing means, there were no difference in the AMH levels, but there were lower mean values for inhibin B (p < .05) and ovarian volume (p < .01) in the CCS group (data not shown). AFC was also lower among CCS compared to controls (p < .05), but FSH showed no difference between CCS and controls.
Correlations of the different ovarian parameters showed the same results for all ages; the one exception was for FSH, which did not correlate to either AFC or ovarian volume among younger CCS (data not shown). With linear regression, there also were no differences between different ovarian parameters comparing the CCS and controls in this younger age group.
ROC curve analysis for primary POI test accuracy for AMH was 0.977, for inhibin B 0.728, for AFC 0.984, and for ovarian volume 0.813. ROC curve analysis for AFC <10 among CCS showed test accuracy for AMH 0.848, for inhibin B 0.786, and for ovarian volume 0.883. Among controls, AFC <10 test accuracy for AMH was 0.815, for inhibin B 0.722, and for ovarian volume 0.746. To conclude, there were no major differences in results in this younger age group.
Discussion
To our knowledge, this is the first study that evaluates how different serum markers correlated to AFC and ovarian volume in a group of female adult CCS and controls. We also tested the accuracy of markers for primary POI and low AFC. Serum levels of AMH, inhibin B and FSH correlated well with AFC and ovarian volume both among CCS and controls, but in women below 40 years, FSH was not reliable as many women had ongoing treatment with HRT or OC. Estradiol did not correlate to either AFC or ovarian volume, although it is recommended in many follow-up programs. AMH was the most accurate serum marker in terms of primary POI and AFC <10 in all ages.
So far, AMH and AFC have demonstrated the best reliability as screening tests of ovarian reserve although there is no single test with 100% sensitivity or specificity [Citation7,Citation9]. AFC requires a transvaginal ultrasound, an examination that young females often experience as discomfort. Therefore, a transvaginal ultrasound is unsuitable for screening. AMH has the advantage of stability through the menstrual cycle [Citation25] as opposed to inhibin B, which has the highest values during the follicular phase [Citation26]. A rise in FSH is a late sign of diminished ovarian reserve, and estradiol levels are not considered reliable [Citation7].
A recent survey compared different follow-up programs for CCS at risk for POI [Citation3]. Besides clinical examination of pubertal development and growth, some programs recommend FSH and estradiol for the screening of primary POI, but no program included AMH when screening for primary POI. These programs use FSH and estradiol as the first test for primary POI after puberty and it was suggested that AMH might be reasonable in those >25 years who have menstrual cycle dysfunction or need assessment of future fertility. Our study showed the very limited value of estradiol for surveillance but found AMH to be a valuable marker of ovarian function.
In two earlier studies, we longitudinally followed AMH before, during and after treatment for childhood cancer [Citation18,Citation19]. Most girls, irrespective of age, had an immediate decline in AMH levels after the start of treatment. In those with transient ovarian dysfunction, an increase in AMH levels was noted up to two years after treatment. Although AMH may increase the first years after the end of cancer treatment and AMH naturally increases up to age 25 years [Citation10], following AMH should be considered even for younger girls at risk of ovarian dysfunction after treatment with alkylating agents or ovarian exposure to radiation. However, more studies are needed. According to one study, persisting low AMH levels at least one year after completed cancer treatment is a risk factor for delayed puberty [Citation14]. In a clinical setting, persisting low AMH levels several years after cancer treatment is a risk factor for absent puberty; in these girls, puberty can be induced at a proper time rather than waiting until there are no signs of puberty, around the age of 13 [Citation3].
We found POI in 13% of CCS; other studies found a POI prevalence of 8–10.9% [Citation2,Citation27]. Compared to previous studies, our CCS were slightly older at examination and the median follow-up time was longer. These objectives might explain the higher prevalence of POI in our cohort, which was smaller but representative regarding childhood cancer diagnoses. In one earlier study, the frequency of primary POI was 17% [Citation28], but it only included patients treated with chemotherapy and/or radiotherapy. Our study included all eligible patients treated for childhood cancer and the patients treated only with surgery.
We found that all but one of the CCS with POI under age 40 years at examination received HRT. However, Chemaitilly et al. 2017 [Citation27] found that only one-third of childhood cancer survivors had ongoing estrogen replacement. The CCS needs to be informed about the risk of POI in case of gonadotoxic treatment regardless of dose and/or abdominal radiotherapy. Follow-up programs are important, but there are still many areas where concordant programs need to be considered.
This study has weaknesses. The data were gathered during a five-year period causing an age discrepancy of 0–3 years between the CCS and the control women regarding time for blood sampling. Because the matching was not made for OC and HRT, the data were analyzed for groups and not paired data. Six different examiners performed the examination with transvaginal ultrasound, with probable interindividual differences. AFC and ovarian volume was not available in 19% and 23% of CCS compared to 4 and 5% of controls. This emphasizes the difficulties with ultrasound examination in the survivor group. We had no information of laboratory results at POI diagnosis but used the definition stated in the introduction. The study group is small, but the examinations were thorough and from our point of view the CCS group is representative for adult Swedish females treated for childhood cancer.
In summary, we found POI in 13% among CCS, slightly more than in other studies. Serum levels of AMH, inhibin B and FSH correlated well with AFC and ovarian volume both among CCS and controls. In women below 40 years, AMH and inhibin B were the most reliable serum markers. AMH showed consistent test accuracy and our study supports AMH to be the most reliable serum marker reflecting ovarian function after cancer treatment. The study suggests AMH as a valuable tool in follow-up programs of female CCS at risk of ovarian insufficiency.
| Abbreviations | ||
| AFC | = | antral follicle count |
| AMH | = | anti-Müllerian hormone |
| AUC | = | area under curve |
| ROC | = | Receiving Operating Characteristics curve |
| CI | = | confidence interval |
| CCS | = | childhood cancer survivors |
| E2 | = | estradiol |
| FSH | = | follicle stimulating hormone |
| HRT | = | hormone replacement therapy |
| HSCT | = | hematopoietic stem cell transplantation |
| OC | = | oral contraceptives |
| POI | = | premature ovarian insufficiency |
Supplemental Material
Download MS Word (60.5 KB)Supplemental Material
Download MS Word (232.5 KB)Supplemental Material
Download MS Word (14.4 KB)Acknowledgments
Research nurse Irene Leijonhufvud is gratefully acknowledged for excellent assistance, administration, and support during the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Green DM, Sklar CA, Boice JD Jr, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Onc. 2009;27:2374–2381.
- Sklar CA, Mertens AC, Mitby P, et al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98:890–896.
- van Dorp W, Mulder RL, Kremer LC, et al. Recommendations for premature ovarian insufficiency surveillance for female survivors of childhood, adolescent, and young adult cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in Collaboration With the PanCareSurFup Consortium. Jco. 2016;34:3440–3450.
- De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376:911–921.
- European Society for Human R, Embryology Guideline Group on POI, Webber L, Davies M, Anderson R, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31:926–937.
- Wasilewski-Masker K, Kaste SC, Hudson MM, et al. Bone mineral density deficits in survivors of childhood cancer: long-term follow-up guidelines and review of the literature. Pediatrics. 2008;121:e705–e713.
- Practice Committee of the American Society for Reproductive M. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103:e9–e17.
- Burger HG, Dudley EC, Hopper JL, et al. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab. 1999;84:4025–4030.
- Scheffer GJ, Broekmans FJ, Looman CW, et al. The number of antral follicles in normal women with proven fertility is the best reflection of reproductive age. Hum Reprod. 2003;18:700–706.
- Lie Fong S, Visser JA, Welt CK, et al. Serum anti-müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97:4650–4655.
- Kelsey TW, Wright P, Nelson SM, et al. A validated model of serum anti-müllerian hormone from conception to menopause. PLoS One. 2011;6:e22024.
- Tobler KJ, Shoham G, Christianson MS, et al. Use of anti-mullerian hormone for testing ovarian reserve: a survey of 796 infertility clinics worldwide. J Assist Reprod Genet. 2015;32:1441–1448.
- Bath LE, Wallace WH, Shaw MP, et al. Depletion of ovarian reserve in young women after treatment for cancer in childhood: detection by anti-Mullerian hormone, inhibin B and ovarian ultrasound. Hum Reprod. 2003;18:2368–2374.
- Lunsford AJ, Whelan K, McCormick K, et al. Antimullerian hormone as a measure of reproductive function in female childhood cancer survivors. Fertil Steril. 2014;101:227–231.
- Lie Fong S, Laven JS, Hakvoort-Cammel FG, et al. Assessment of ovarian reserve in adult childhood cancer survivors using anti-Mullerian hormone. Hum Reprod. 2008;24:982–990.
- van Beek RD, van den Heuvel-Eibrink MM, Laven JS, et al. Anti-Mullerian hormone is a sensitive serum marker for gonadal function in women treated for Hodgkin's lymphoma during childhood. J Clin Endocrinol Metab. 2007;92:3869–3874.
- Miyoshi Y, Ohta H, Namba N, et al. Low serum concentrations of anti-Müllerian hormone are common in 53 female childhood cancer survivors. Horm Res Paediatr. 2013;79:17–21.
- Morse H, Elfving M, Lindgren A, et al. Acute onset of ovarian dysfunction in young females after start of cancer treatment. Pediatr Blood Cancer. 2013;60:676–681.
- Morse H, Elfving M, Turkiewicz A, et al. Severe gonadotoxic insult manifests early in young girls treated for Ewing sarcoma. Medicine (Baltimore). 2016;95:e4512.
- Hansen KR, Hodnett GM, Knowlton N, et al. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95:170–175.
- Xiao B, Zeng T, Wu S, et al. Effect of levonorgestrel-releasing intrauterine device on hormonal profile and menstrual pattern after long-term use. Contraception. 1995;51:359–365.
- Bobjer J, Bogefors K, Isaksson S, et al. High prevalence of hypogonadism and associated impaired metabolic and bone mineral status in subfertile men. Clin Endocrinol (Oxf). 2016;85(2):189–195.
- Majumder K, Gelbaya TA, Laing I, et al. The use of anti-Müllerian hormone and antral follicle count to predict the potential of oocytes and embryos. Eur J Obstet Gynecol Reprod Biol. 2010;150:166–170.
- Heyman M, Gustafsson G, Kogner P, et al. Stockholm. Childhood Cancer Incidence and Survival in Sweden 1984–2010. https://wwwccegkise/documents/ChildhoodCancerIncidenceandSurvivalinSweden1984_2010pdf. 2013.
- La Marca A, Stabile G, Artenisio AC, et al. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod. 2006;21:3103–3107.
- Yding Andersen C. Inhibin-B secretion and FSH isoform distribution may play an integral part of follicular selection in the natural menstrual cycle. Mol Hum Reprod. 2017;23:16–24.
- Chemaitilly W, Li Z, Krasin MJ, et al. Premature ovarian insufficiency in childhood cancer survivors: a report from the St. Jude Lifetime Cohort. J Clin Endocrinol Metab. 2017;102:2242–2250.
- Larsen EC, Muller J, Schmiegelow K, et al. Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab. 2003;88:5307–5314.
