Abstract
Background: In randomised controlled trials, men with advanced, non-metastatic prostate cancer (PCa) treated with anti-androgen monotherapy (AA) had similar all-cause mortality as men treated with gonadotropin-releasing hormone (GnRH) agonists. Using real-world evidence (i.e., observational data), we aimed to further assess the difference in mortality between these two drug categories.
Material and Methods: We emulated a trial using data from Prostate Cancer data Base Sweden 3.0. We specifically focused on men diagnosed in 2006–2012 with high-risk PCa who had no distant metastasis. They either received primary hormonal therapy with AA (n = 2078) or GnRH agonists (n = 4878) who were followed for a median time of 5 years. Risk of death from PCa and other causes was assessed using competing risk analyses and Cox proportional hazards regression analyses, including propensity score matching.
Results: The cumulative 5-year PCa mortality was lower for men treated with AA (16% [95% confidence interval, CI, 15–18%]) than men treated with GnRH agonists (22% [95% CI 21–24%]). The 5-year other cause mortality was also lower for men on AA (17% [95% CI 15–19%] compared to men on GnRH agonists (27% [95% CI 25–28%]). In regression analyses, the risk of PCa death was similar, GnRH agonists versus AA (reference), hazard ratio (HR) 1.08 (95% CI 0.95–1.23), but the risk of death from all causes was higher for men on GnRH agonists, HR 1.23 (95% CI 1.13–1.34). Consistent results were seen in the propensity score-matched cohort.
Conclusion: Our results indicate that the use of AA as primary hormonal therapy in men with high-risk non-metastatic PCa does not increase PCa-specific mortality compared to GnRH. Using AA instead of GnRH agonists may result in shorter time on/exposure to GnRH-treatment, which may reduce the risk of adverse events associated with this treatment.
Introduction
Radiotherapy with adjuvant hormonal therapy is the recommended treatment for men with high-risk prostate cancer (PCa) with no distant metastasis [Citation1]. However, a substantial number of these men start primary hormonal therapy without radiotherapy, especially men with prostate specific-antigen (PSA) levels above 50 ng/mL and/or locally advanced PCa (clinical local stage T3-4) [Citation2,Citation3]. Moreover, around 20% of men diagnosed with localised PCa who received primary curative treatment will require hormonal therapy within 10 years [Citation4].
The two main types of hormonal therapy for high-risk PCa with no distant metastasis are anti-androgen monotherapy (AA) and gonadotropin-releasing hormone (GnRH) agonists [Citation5]. Their mechanism of action is very different. AAs competitively bind to androgen receptors, resulting in a decline in testosterone biosynthesis [Citation6,Citation7]. In contrast, GnRH agonists bind GnRH receptors on pituitary gonadotropin-producing cells, which causes a temporary release of LH and FSH. Thus, AA have less effect on a reduction of testosterone levels in the circulation as compared to GnRH agonists [Citation8].
The adverse events profile for these two treatments is different, mainly due to these different effects on circulating testosterone levels. For men on AA, the most frequent adverse events are breast pain and gynaecomastia (due to conversion of testosterone into estradiol), while liver toxicity is a rare but serious event [Citation9,Citation10]. For men on GnRH agonists, the most common adverse events are hot flushes, weight gain, loss of libido and erectile dysfunction [Citation8]. Additionally, GnRH agonists are associated with a number of long-term adverse metabolic effects including bone loss and increased risk of fractures [Citation11], cardiovascular disease [Citation12,Citation13], diabetes mellitus type 2 [Citation12] and possibly dementia [Citation14]. Low levels of androgens have been shown to increase levels of low-density lipoprotein (LDL), triglycerides and insulin – all risk factors of ardiovascular disease [Citation15]. Furthermore, testosterone may be protective against the development of atheromatous plaques by causing coronary artery dilation and inhibiting the effect of pro-inflammatory cytokines [Citation16].
The current EAU-ESTRO-SIOG guidelines do not recommend AA with bicalutamide 150 mg/daily as standard of care for men with high-risk PCa with no distant metastasis [Citation1]. This recommendation is based on a Cochrane review, which concluded that AA in men with advanced PCa is less effective than castration in terms of overall survival [Citation17]. However, this review included studies with different types of AA and different dosages. Furthermore, in the subgroup analysis of men with advanced, non-metastatic PCa treated with bicalutamide 150 mg/daily, overall survival was similar to castration, in line with two randomised controlled trials (RCTs) [Citation18,Citation19]. These two RCTs specifically compared the clinical effectiveness as well as adverse events of bicalutamide 150 mg/daily versus GnRH agonists or maximal androgen blockade (GnRH agonist combined with continuous AA). Although the statistical requirement for non-inferiority was not met, survival was similar between the group of men treated GnRH agonists and those treated with bicalutamide 150 mg/daily. Based on these trials, the European Medicines Agency subsequently approved this AA for use in men with advanced, non-metastatic PCa [Citation20].
Even though RCTs are considered the gold standard for evaluating the effectiveness of interventions, observational data, also known as real-world data, is an important addition to RCTs in clinical decision making provided that confounding by indication is appropriately handled [Citation21]. Compared to patients outside RCTs, participants in RCTs are often highly selected with smaller cancer burden, and elderly patients and those with comorbidities are frequently excluded [Citation22,Citation23]. Thus, additional evidence from observational studies in support of results from RCTs is needed to show the external validity of these results.
When PCa cells escape the control of AA leading to disease progression, men on AA will switch to GnRH agonists [Citation24]. Consequently, men who start on AA are less exposed to the broad/severe systemic and metabolic side effects of GnRH agonists than those who start on GnRH agonists. Hence, evaluating possible differences in PCa specific mortality and overall mortality amongst those starting on AA versus those starting on GnRH agonists is important.
We aimed to further investigate the potential difference in mortality between primary AA and GnRH agonists, by supplementing existing RCT evidence using real-world data.
Patients and methods
Data resource
The current study is based on data from Prostate Cancer data Base Sweden (PCBaSe) 3.0, which contains information on cancer characteristics and primary treatment from the National Prostate Cancer Register (NPCR) of Sweden [Citation24]. Data on comorbidity were obtained from the Patient Registry, data on educational level, income and marital status from the LISA database, and cause and date of death from the National Cause of Death Registry.
Target trial
To ensure clinically meaningful results by use of real-world data, we used the ROBINS-I tool to emulate a target trial for risk of death in men on GnRH agonists or AA [Citation25]. A target trial is a pragmatic trial that resembles a hypothetical RCT using observational data. Such an approach is considered useful when designing an observational study to assess the effects of different types of drugs. The ROBINS-I tool was used to assess potential biases before, during and after intervention in this study (See Supplemental Appendix) [Citation25]. This tool was specifically designed to evaluate the risk of bias in comparative effectiveness studies that do not use randomisation to allocate individuals to comparison groups.
For this study, we selected all men from PCBaSe aged ≤90 years who were diagnosed with high-risk or regionally metastatic PCa, i.e., clinical local stage T3 or higher and/or PSA 20 ng/mL or higher and/or Gleason Grade Group 4–5 and/or N1 and no distant metastases. We specifically selected men diagnosed in 2006–2012 who received AA or GnRH agonists as primary therapy. The study then set out to compare AA monotherapy (150 mg/daily) with GnRH agonists. The large majority of men on AA received bicalutimide, for which adherence has previously been shown to be very good [Citation26]. This information, as well as conversion from AA to GnRH agonists, was verified through data on filled prescriptions in the Prescribed Drug Registry. In case of disease progression, the switch to GnRH agonists was allowed and handled based on the intention-to-treat approach.
Follow-up was then calculated from the date of PCa diagnosis until death, emigration or date of censoring, whichever event came first. End of follow-up was 31 December 2015 for those analyses focused on PCa mortality, and 31 December 2016 for those analyses focused on all-cause mortality.
The Research Ethics Board at Umeå University Hospital approved the study.
Imputation
To handle missing data for potential confounding factors, we performed multiple imputation using the method of Chained Equations and the MICE package [Citation27]. Data were missing for the following variables: mode of detection (3% missing), T stage (1% missing), N stage (0.3% missing), Gleason Grade Groups (5% completely missing and 1% not differentiating between GGG1 or GGG2), percent positive biopsies (25% missing), PSA (1% missing) and education (1% missing).
Moreover, to account for differential misclassification of M1 disease, which included Mx when no bone scan was performed (prior to 2011), we conducted an inputation of M status (50% were recorded as Mx). In our imputation models, we included information from all men with intermediate-, high-risk, regionally metastatic and metastatic disease who were treated with primary hormonal therapy (Supplemental Table 1). The number of multiple imputations was set to five. All subsequent analyses were conducted using the imputed data sets, for men originally categorised with high-risk or regionally metastatic PCa, but excluding those with imputed M1 disease. A sensitivity analysis ignoring imputed M1 disease was also performed.
Statistical methods
First, we created cumulative incidence graphs showing PCa-specific death, overall death, and conversion from AA to GnRH agonists. Then, we conducted both traditional Cox proportional hazards regression analyses as well as propensity score-matched analyses within the target trial population. The latter allowed for a detailed assessment of our real-world data.
Cox proportional hazards regression analyses
We conducted uni- and multivariate Cox proportional hazards regression analyses for death of PCa and death from all causes using age as a timescale, whilst adjusting for year of diagnosis (continuous), mode of detection (categorical), T stage (categorical), Gleason Grade Groups (categorical), proportion positive biopsy cores (modelled as an interaction with T stage in men not diagnosed following TUR-P with two spine knots), PSA at diagnosis (categorical), bone imaging performed (dichotomised), Charlson comorbidity index (categorical), marital status (categorical) and education level (categorical). Results are presented as Hazard ratios (HR) with 95% confidence intervals (CI).
Propensity score matched analyses
We then conducted additional Cox model analysis based on propensity score matching for the type of hormonal therapy. Propensity score matching was done with the MatchIt package for R using a caliper of 0.1 and included the covariates enumerated above. Subsequent multivariable Cox proportional hazards regression analyses were performed adjusting for the covariates used to perform the propensity score matching. Finally, we created 1-Kaplan-Meier estimates of PCa-specific and overall survival for the propensity score matched groups.
All statistical analysis was performed with R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The study population consisted of 2078 men on AA and 4878 men on GnRH agonists as primary hormonal therapy (). The median follow-up was 4.7 years, representing a total of 28,315 person-years. Men treated with AA were younger, diagnosed in more recent calendar years, had less adverse cancer characteristics and had higher education level, compared to men treated with GnRH agonists (). Across the 5 imputed datasets, the number with imputed M1 disease ranged from 7 to 20 (mean =14) amongst those treated with AA and from 109 to 162 (mean =140) amongst those treated with GnRH agonists. In total, 765 (37%) men converted from AA to GnRH agonists, with a median time of exposure to AA of 4.3 years (95% CI 4.1–4.6) ().
Figure 2. First event of conversion from AA to GnRH agonist, death from other causes or death from PCa assessed in competing risk analyses among men who started on anti-androgen monotherapy (AA) in competing risk analyses.
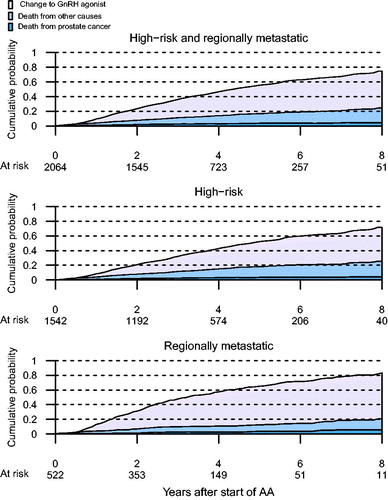
Table 1. Baseline characteristics of men in PCBaSe 3.0 diagnosed with high-risk and regionally metastatic prostate cancer in 2006–2012 and treated with anti-androgen monotherapy or GnRH agonists.
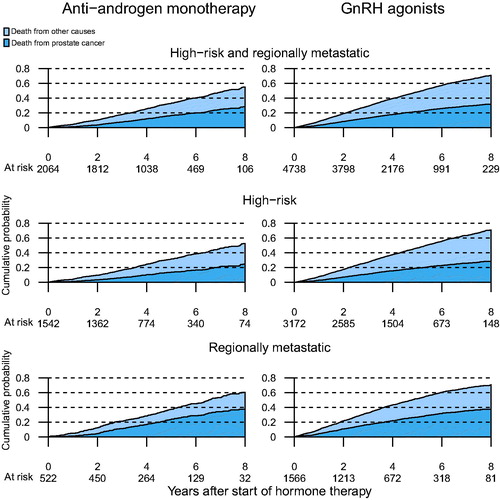
The 5-year crude cumulative incidence of PCa mortality for men on AA was lower than men on GnRH agonists (AA 16% [95% CI 15–18%] vs. GnRH agonists 22% [95% CI 21–24%]). The 5-year cumulative mortality incidence of other causes than PCa was also lower for men on AA than men on GnRH agonists (AA 17% [95% CI 15–19%] and GnRH agonists 27% [95% CI 25–28%]) ().
Figure 3. Cumulative probability of death from PCa or death from other causes for men on anti-androgen monotherapy (AA) and men on GnRH agonists.
Using the traditional Cox proportional hazards regression analyses, we found that men who received GnRH agonists had a similar risk of death from PCa as men on AA, HR 1.08 (95% CI 0.95–1.23), but a higher risk of death from all causes, HR 1.23 (95% CI 1.13–1.34) (). Stratification by PCa risk category revealed similar results, with the exception of no difference in death from all causes in men with regional metastatic PCa, HR 1.09 (95% CI 0.94–1.26).
Table 2. Risk of death from prostate cancer or death from all causes in men on primary anti-androgen monotherapy or GnRH agonists.
Following propensity score matching, a total of 1972–1976 men were identified in each treatment group in the 5 imputed datasets. Similar to the results of the traditional multivariable Cox analyses, men on GnRH agonists had a similar risk of death from PCa as men on AA, HR 1.09 (95% CI 0.94–1.27), but a higher risk of death from all causes, HR 1.25 (95% CI 1.14–1.37) (). Stratification by PCa risk category revealed similar results, again with the exception of men with regionally metastatic PCa for whom there was no difference in deaths from all causes. shows the 1-Kaplan-Meier curves for PCa-specific death and all deaths. While there was no statistically significant difference in 5-year PCa-specific mortality, the 5-year all-cause mortality was lower for men on AA (32% [95% CI 30–35%]) than for men on GnRH agonists (42% [95% CI 39–45%)].
Figure 4. Probability of PCa death and death from all causes following propensity score matched for men on anti-androgen monotherapy (AA) and GnRH agonists, assessed with 1-Kaplan-Meier estimates.
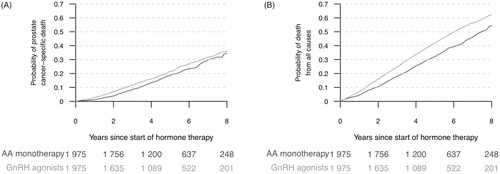
Table 3. Risk of death from prostate cancer or death from all causes for men on primary GnRH agonists or anti-androgen monotherapy (AA, reference in analyses) following propensity score matching.
In a sensitivity analysis, we evaluated the effect of inclusion of imputed M1 disease in our regression models. This showed that our exclusion of M1disease attenuated the difference in risk of death between AA and GnRH agonsits, which highlights the importance of having correct staging information.
Discussion
In this register-based, observational study of men with high-risk PCa with no distant metastasis, treated with primary hormonal therapy, men on AA had similar PCa mortality and lower all-cause mortality than men on GnRH agonists.
There are two ways to interpret our results. Firstly, these findings could represent the true effect of two treatments which are known to have different mechanisms of action and different side-effect profiles. Alternatively, our results could be due to the effect of bias, given the observational nature of this study design. Even though RCTs are considered the gold standard for comparisons of treatments, it has now been recognised that clinical trials may fail to show clinical effectiveness [Citation23]. The guidelines from the European GetReal consortium (‘incorporating real-life data into drug development’) specifically recommend considering evidence from pragmatic trials and non-randomised studies to improve applicability of treatment effect estimates, inform disconnected or scarce networks of evidence, identify patient populations that will likely receive the drug after launch, and to improve relevant to decision/policy makers and patients [Citation22]. In the current register-based, observational study, all men with relevant cancer characteristics were included regardless of other characteristics, with the exception of very high age (older than 90 years of age) and a previous cancer diagnosis.
A general limitation of observational studies is the lack of randomisation and possible impact of bias caused by treatment selection. Hence, to evaluate the potential risk of bias, we used the ROBINS-I tool (Supplemental Appendix 1). After carefully assessing each of the domains outlined in the ROBINS-I tool, we concluded that the overall risk of bias was moderate. Therefore, our study provides sound evidence for a non-randomised study, but cannot be considered comparable to a well-performed RCT. Consequently, our results are deemed to be hypothesis-generating.
In the current study, men managed with AA were younger, diagnosed in more recent calendar years, had less comorbidities and had less adverse cancer characteristics compared to men treated with GnRH agonists. We adjusted for these differences in the traditional Cox regression analyses and found similar risk of PCa death in men on AA and men on GnRH agonists. However, we also observed a lower all-cause mortality among men with high-risk PCa treated with AA, while men with regional metastatic PCa had similar risk of deaths from all causes, in line with results from previous RCTs [Citation18,Citation19]. Unmeasured confounders are likely to account for some of this difference.
To further adjust for differences between treatment groups, we identified propensity score-matched groups of men on AA and men on GnRH agonists. A well-matched propensity score analysis is based on balanced baseline patient characteristics and excludes exposed participants who had no comparable unexposed participant and vice versa [Citation28]. Propensity score matching does not assume linearity in the relationship between the propensity and outcome and allows for simple, transparent analyses. It provides a better balance of covariates between exposed and unexposed groups compared to other matching strategies in datasets with many covariates [Citation29]. The propensity score-matched analyses revealed similar results as the traditional multivariable Cox regression analyses – which is not surprising given the number of detailed covariates available in this large dataset [Citation30].
As previously mentioned, men who started on AA switched to GnRH agonists after about 4.3 years. Our results showing no difference in PCa-specific mortality and lower overall mortality in the AA group, suggest that AA can be considered as an initial alternative therapy for men with locally advanced PCa. Nevertheless, it is important to note that our study design used intention to treat rather than per protocol analysis. The former ensures comparability between groups that are obtained through randomisation (propensity score matching in this particular setting) and maintains sample size. Hence, one of the limitations of our study is potential dilution of the treatment effect estimation due to noncompliance [Citation31].
The main limitation of our study was the non-random allocation to type of hormonal therapy, with ensuing channelling bias of younger and healthier men with less advanced cancer to receive AA. However, we used a robust study design with application of propensity score matching with fairly well-balanced baseline characteristics in the two treatment arms. Yet this method is based on measured variables, thus residual confounding is likely to be present as in most observational studies. Strengths of our study include the nationwide, population-based cohort of men with comprehensive data from a clinical cancer register with documented high data quality as well as several other high-quality health care registers [Citation32,Citation33], a setting that thus provides strong real-world data.
Conclusion
Using a hypothetical trial in a real-world setting, our results indicate that in men with high-risk PCa with no distant metastasis, PCa-specific mortality is similar for those treated with AA and those treated with GnRH agonists. However, all-cause mortality was lower for men taking AA compared with men on GnRH agonists. Starting on AA instead of GnRH agonists may result in less exposure to GnRH agonists and hence potentially less risk of adverse events.
Acknowledgments
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden (NPCR) steering group: Pär Stattin (chairman), Anders Widmark, Camilla Thellenberg Karlsson, Ove Andrén, Ann-Sofi Fransson, Magnus Törnblom, Stefan Carlsson, Marie Hjälm-Eriksson, David Robinson, Mats Andén, Jonas Hugosson, Ingela Franck Lissbrant, Maria Nyberg, Ola Bratt, Lars Egevad, Calle Waller, Stig Hanno, Olof Akre, Per Fransson, Eva Johansson, Fredrik Sandin, and Karin Hellström.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol. 2017;71:630–642.
- Guy D, Ghanem G, Loblaw A, et al. Diagnosis, referral, and primary treatment decisions in newly diagnosed prostate cancer patients in a multidisciplinary diagnostic assessment program. Can Urol Assoc J. 2016;10:120–125.
- Thomsen FB, Mikkelsen MK, Hansen RB, et al. Clinical characteristics and primary management of patients diagnosed with prostate cancer between 2007 and 2013: status from a Danish primary referral center. Acta Oncol. 2016;55:1456–1460.
- Lycken M, Garmo H, Adolfsson J, et al. Patterns of androgen deprivation therapies among men diagnosed with localised prostate cancer: a population-based study. Eur J Cancer. 2014;50:1789–1798.
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2017;71:618–629.
- Albertsen PC, Klotz L, Tombal B, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65:565–573.
- Reid P, Kantoff P, Oh W. Antiandrogens in prostate cancer. Invest New Drugs. 1999;17:271–284.
- Crawford ED, Shore ND, Moul JW, et al. Long-term tolerability and efficacy of degarelix: 5-year results from a phase III extension trial with a 1-arm crossover from leuprolide to degarelix. Urology. 2014;83:1122–1128.
- Iversen P, McLeod DG, See WA, et al. Antiandrogen monotherapy in patients with localized or locally advanced prostate cancer: final results from the bicalutamide Early Prostate Cancer programme at a median follow-up of 9.7 years. BJU Int. 2010;105:1074–1081.
- Nguyen PL, Alibhai SM, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67:825–836.
- Thorstenson A, Bratt O, Akre O, et al. Incidence of fractures causing hospitalisation in prostate cancer patients: results from the population-based PCBaSe Sweden. Eur J Cancer. 2012;48:1672–1681.
- Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. Jco. 2006;24:4448–4456.
- Van Hemelrijck M, Garmo H, Holmberg L, et al. Absolute and relative risk of cardiovascular disease in men with prostate cancer: results from the Population-Based PCBaSe Sweden. J Clin Oncol. 2010;28:3448–3456.
- Nead KT, Gaskin G, Chester C, et al. Association between androgen deprivation therapy and risk of dementia. JAMA Oncol. 2017;3:49–55.
- Dockery F, Bulpitt CJ, Agarwal S, et al. Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci. 2003;104:195–201.
- Malkin CJ, Pugh PJ, Jones RD, et al. Testosterone as a protective factor against atherosclerosis–immunomodulation and influence upon plaque development and stability. J Endocrinol. 2003;178:373–380.
- Kunath F, Grobe HR, Rucker G, et al. Non-steroidal antiandrogen monotherapy compared with luteinising hormone-releasing hormone agonists or surgical castration monotherapy for advanced prostate cancer. Cochrane Database Syst Rev. 2014 Jun 30;(6): CD009266.
- Boccardo F, Rubagotti A, Barichello M, et al. Bicalutamide monotherapy versus flutamide plus goserelin in prostate cancer patients: results of an Italian Prostate Cancer Project study. J Clin Oncol. 1999;17:2027–2038.
- Iversen P, Tyrrell CJ, Kaisary AV, et al. Bicalutamide monotherapy compared with castration in patients with nonmetastatic locally advanced prostate cancer: 6.3 years of followup. J Urol. 2000;164:1579–1582.
- EM Agency. Bicalutamide 150 mg; 2007 [updated 2007 May 24; cited 2018 Feb 01]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Bicalutamide_150_mg/human_referral_000116.jsp
- Faria R, Alava MH, Manca A, Wailoo AJ. National Institute for Health and Care: the use of observational data to inform estimates of treatment effectiveness in technology appraisal: methods for comparative individual patient data; 2015. Available from: http://nicedsu.org.uk/technical-support-documents/observational-data-tsd/
- Egger M, Moons KG, Fletcher C. GetReal: from efficacy in clinical trials to relative effectiveness in the real world. Res Synth Methods. 2016;7:278–281.
- Unger JM, Barlow WE, Martin DP, et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. 2014;106:dju002.
- Van Hemelrijck M, Garmo H, Wigertz A, et al. Cohort Profile Update: The National Prostate Cancer Register of Sweden and Prostate Cancer data Base – a refined prostate cancer trajectory. Int J Epidemiol. 2016;45:73–82.
- Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
- Grundmark B, Garmo H, Zethelius B, et al. Anti-androgen prescribing patterns, patient treatment adherence and influencing factors; results from the nationwide PCBaSe Sweden. Eur J Clin Pharmacol. 2012;68:1619–1630.
- Van Buuren S. Flexible imputation of missing data. New York: CRC press; 2012.
- Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98:253–259.
- Gu XS, Rosenbaum PR. Comparison of multivariate matching methods: structures, distances, and algorithms. J Comput Graph Stat. 1993;2:405–420.
- Vickers AJ, Sjoberg DD, European U. Guidelines for reporting of statistics in European Urology. Eur Urol. 2015;67:181–187.
- Ranganathan P, Pramesh CS, Aggarwal R. Common pitfalls in statistical analysis: intention-to-treat versus per-protocol analysis. Perspect Clin Res. 2016;7:144–146.
- Tomic K, Berglund A, Robinson D, et al. Capture rate and representativity of The National Prostate Cancer Register of Sweden. Acta Oncol. 2015;54:158–163.
- Tomic K, Sandin F, Wigertz A, et al. Evaluation of data quality in the National Prostate Cancer Register of Sweden. Eur J Cancer. 2015;51:101–111.