Abstract
Background: In luminal A-like breast cancer, the indication for adjuvant chemotherapy still is largely based on lymph nodestatus. Sentinel node (SN) positive patients who are no longer recommended an axillary lymph node dissection (ALND) risk undertreatment, especially if they have ≥4 lymph node metastases (LNMs).
Aim: To quantify the group of SN positive luminal A-like patients with ≥4 LNMs and to investigate if the ratio between positive SNs and examined SNs (SN ratio) can provide information of the axillary tumor burden.
Material and methods: Nearly 370 patients between 2014-2016 in Western Sweden with a clinically node-negative invasive breast cancer treated with both SNB and subsequent ALND were included. SNB findings were compared to ALND findings and the ability of the SN ratio to describe the axillary tumor burden was evaluated using multivariable analysis.
Results: In total,17.6% of patients with luminal A-like tumors had ≥4 LNMs. The SN ratio was statistically significant for predicting ≥4 LNMs among luminal A-like patients (p = .013, OR 1.89 (95% CI 1.14–3.12) per 0.1 increment in SN ratio).
Conclusions: One sixth of patients with SN positive luminal A-like tumors risk undertreatment. The SN ratio may aid in identifying many of these patients.
Introduction
The prevalence of >2 positive SNs or extracapsular growth seems to be able to identify breast cancer patients with a high risk of additional non-sentinel node (NSN) metastases (72%) while the risk for patients with one to two positive sentinel nodes (SNs) is much lower [Citation1]. The current literature therefore suggests that many patients are overtreated when they undergo axillary lymph node dissection (ALND) based on one to two positive SNs and it has become a widespread international standard to avoid axillary dissection in such cases [Citation2].
Luminal A is the subtype with the best prognosis. It is characterized by a low proliferation rate, a high expression of hormone receptors and a low expression of HER2. Having a low proliferation rate makes this subtype less responsive to chemotherapy and several studies have questioned the benefit of chemotherapy in this patient group [Citation3–5]. There is an inverse relationship between chemotherapy responsiveness and estrogen receptor expression as the latter serves as an indirect measure of other biomarkers related to tumor growth [Citation6–8]. However, having ≥4 LNMs is strongly prognostic of a more advanced and aggressive disease also among ER positive, HER2 negative breast cancer patients with macrometastases in SN [Citation9–11]. Studies have shown that high-risk hormone receptor positive breast cancer patients with ≥4 LNMs benefit from chemotherapy [Citation12–16] although there are also studies which have failed to demonstrate this specifically for luminal A patients [Citation3,Citation4]. A limitation of the studies that did not show a therapeutic benefit for luminal A patients is that they did not primarily focus on patients with ≥4 LNMs.
The St Gallen consensus expert panel recommends adjuvant chemotherapy to luminal A patients with ≥4 LNMs [Citation17]. This means that the ALND may provide crucial information for luminal A patients who have one to two positive SNs but ≥4 total LNMs (SNs + NSNs).
Our hypothesis was that 5–10% of luminal A-like patients will risk undertreatment with adjuvant chemotherapy if the indication for ALND is set to >2 positive SNs. Our aim was to identify in a population-based registry study the proportion of patients with a positive SN and luminal A-like breast cancer that after ALND had ≥4 LNM and to identify predictive factors for this group of patients.
Material and methods
Study population
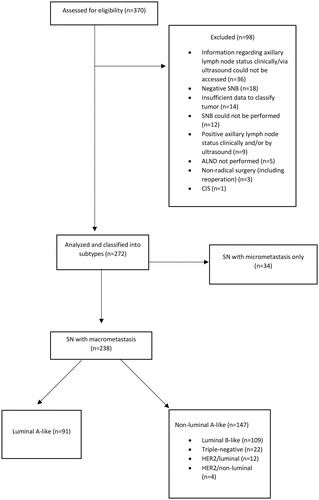
In Western Sweden, breast cancer surgery is performed at five breast cancer units. Data is stored in the Swedish Quality Registry for Breast Cancer on an INCA platform (national cancer IT platform). The register is population-based and contains information of breast cancer patients regarding the number of positive SNs (micrometastases and macrometastases), the number of LNMs from the ALND, the date of the breast cancer diagnosis, the responsible hospital and the personal identification number, unique for each patient. The inclusion criteria were patients registered with: (1) a positive SNB followed by an ALND (2) a clinically negative axilla (3) breast cancer surgery performed in Western Sweden between the years 2014–2016.
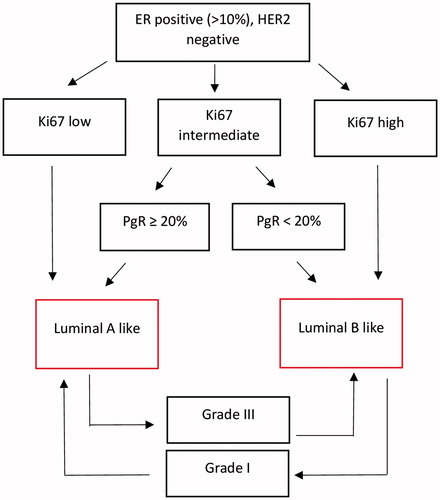
In total, 370 patients met the inclusion criteria and were retrieved from the register and further reviewed in the medical record system for information regarding ER, PgR, Ki67, HER2, grade, tumor size, radicality of surgery, other cancers, axillary lymph node status (clinically and via ultrasound) and recurrence or death caused by the breast cancer. Patients who had undergone ALND despite having a negative SNB were excluded. Patients with insufficient data for tumor classification, patients with clinically/ultrasound positive axilla and patients whose surgery was not radical were also excluded (). According to the Swedish national guidelines, the sentinel nodes were defined as either a hot and blue node, a blue node with blue afferent lymphatic tracts or a hot node with more than 10% of the activity of the hottest node. Patent blue dye and a technetium-99m albumin colloid were used. The luminal tumors were then classified as luminal A-like or luminal B-like based on the St Gallen consensus meeting 2013 [Citation18] and Ki-67 analysis as proposed by Maisonneuve et al [Citation19] while also taking into account the histologic grade [Citation20] using data from the Swedish Quality Registry for Breast Cancer () [Citation19]. Lab-specific cutoffs were used for Ki-67 with the following percental distribution (according to the current Swedish breast cancer guidelines [Citation21]): (1) low; 34% of cases (2) intermediate; 24% of cases (3) high; 42% of cases. The sub-group of patients with luminal A-like tumors was analyzed regarding results from SNB and ALND. Positive HER2 status was defined as immunohistochemistry (IHC) 3+ or IHC 2+ and in situ hybridization (ISH) ratio ≥2 or IHC 2+ and >4 HER2 copies.
Figure 1. Classification of luminal tumors based on ER expression, PgR expression, Ki67 (lab specific cutoffs) and histologic grade.
The study was approved by the Regional Ethical Review Board in Göteborg (Dnr 145-18).
Statistical methods
The ratio of positive SNs compared to the total amount of examined SNs and its ability to predict a tumor burden of ≥4 LNMs was analyzed using a binary logistic regression analysis which also included other known risk factors for having ≥4 LNMs such as SN macrometastases, tumor size and grade. A p value of <.05 was considered statistically significant. The analysis was performed for the luminal A-like group and the nonluminal A group.
Results
In total, 272 patients met the inclusion criteria and were included in the study (). Thirty-four of these had SN micrometastases only and were excluded from further analysis leaving 238 patients with at least one macrometastasis in the SN, of which 91 had a luminal A-like tumor.
The amount of positive SNs varied between 1-4 and the total amount of LNMs after ALND varied between 1 and 23. Patient characteristics are presented in .
Table 1. Patient characteristics.
Sixteen of the luminal A-like patients (17.6%) had ≥4 LNMs. Of these, 2 had >2 positive SNs. Hence, 15.4% of luminal A-like patients had one to two positive SNs but ≥4 LNMs. The relationship between the SN macrometastasis ratio and LNMs among luminal A-like patients can be seen in . Most luminal A-like patients had 1/1 positive SN and 16.3% of these had ≥4 LNMs. The only patients in the group with only one positive SN and ≥4 LNMs were the ones where only one SN had been removed (resulting in a ratio of 1/1). In the group with 2/2 positive SNs, 71.4% had ≥4 LNMs (). In the multivariable analysis for the luminal A-like group, both the number of SN macrometastases (p = .007) and the SN ratio (p = .013) were significant in predicting ≥4 LNMs. Tumor size, grade or age however, were not statistically significant (p = .065, p = .24 and p = .14 respectively) ().
Table 2. SN ratio (number of positive SNs divided by number of examined SNs) and total axillary tumor burden after ALND among luminal A-like patients separated by the number of positive SNs.
Table 3. Multivariable analysis for luminal A-like tumors in predicting a high axillary tumor burden (≥4 LNMs) after ALND.
In the nonluminal A-group, the ability of the SN macrometastasis ratio and the number of SN macrometastases to predict ≥4 LNMs was statistically significant (p = .01 and p = .004 respectively) (). With all subgroups combined, the predicted probability of having ≥4 LNMs was 18.5% for patients with a ratio of 1/1 and 1.7% for patients with a ratio of 1/2. Patients with a ratio of 2/2 were predicted to have a 68.1% risk of having ≥4 LNMs while the risk for patients with a ratio of 2/3 was predicted to be 19.9%.
Table 4. Multivariable analysis for non-luminal A-like tumors in predicting a high axillary tumor burden (≥4 LNMs) after ALND.
Discussion
Our results suggest that around one in six patients with a luminal A-like tumor and a positive SN have ≥4 LNMs and would be recommended chemotherapy if an ALND was performed. The vast majority of these patients (87.5%) had <2 positive SN macrometastases.
According to the current guidelines where adjuvant chemotherapy is indicated for patients with a luminal A-like tumor with ≥4 LNMs, the results of the ALND can change the treatment recommendations regarding chemotherapy. This makes an accurate surrogate marker crucial for optimal treatment of this subgroup. The majority (53.8%) of luminal A-like patients in our study had 1/1 positive SN macrometastasis and 53.1% of these had no additional LNMs (data not shown) which indicates a bad specificity and a significant overtreatment in the majority of cases when the indication for ALND is set to ≥1 positive SNs.
SNB results in a false negative SN in 10% of the cases [Citation22–24] and in most cases with a solitary positive SN there are no additional LNMs [Citation25]. Factors that predict tumor engagement of NSNs are the number of positive SNs, ≥3 SNs removed, macrometastases in SNs, a larger percentage of the SN occupied by metastases, lymphovascular invasion, tumor size, extracapsular growth, higher histologic grade and estrogen receptor negativity [Citation25–27]. The SN macrometastasis ratio was predictive of axillary tumor burden in our study. This was true for the luminal A-like group (p = .013) and for the non-luminal A-like group (p = .01). Luminal A-like patients with a high ratio (1/1 or 2/2) had a higher risk of having ≥ 4 LNMs. These results are in line with findings from previous studies [Citation26,Citation28,Citation29]. A low SN ratio among patients with one to two positive SNs seems to reliably predict a low axillary tumor burden. Our results further suggest that identifying patients with a high axillary tumor burden using >2 positive SNs as a surrogate marker is an unsensitive method as only a small minority of patients with ≥4 LNMs were found to have >2 positive SNs in our study.
The amount of removed SNs varied between one and eight with a median value of one which is lower than what is optimal to prevent false negatives. The procedure of localizing the SNs might have varied between different hospitals. Among patients with fewer removed SNs, it could have been evident to the surgeon that they contained metastases providing an indication for ALND according to the Swedish guidelines and making it unnecessary to remove further SNs. Perhaps the surgeon would have identified and removed more SNs if the indication for ALND had been >2 positive SNs. In the study conducted by Dengel et al, the SNB + ALND group (having >2 positive SNs) had a mean of 5 SNs removed compared to 3 in the SNB only group. The authors speculate that this might be due to the surgeon finding abnormal lymph nodes intraoperatively and having to document >2 positive SNs in order to be able to perform the ALND [Citation1]. Our results suggest that analyzing multiple SNs, if possible, is not only important in order to minimize the false negative rate [Citation30] but also to provide an accurate method of distinguishing patients with <4 LNMs, which is especially important for patients with a luminal A-like tumor for whom adjuvant chemotherapy is not indicated. In the future, gene expression profiling of breast cancer patients might serve as a more accurate method of identifying patients with hormone sensitive tumors whom could benefit from adjuvant chemotherapy [Citation31].
The study consisted of a relatively small group of patients and larger studies are needed to confirm the results. There is also the possibility that the methodology of the SNB differed between different hospitals which could make the findings regarding the SN ratio hard to generalize. Lastly, the group that could benefit from more accurate SN predictions is the luminal A-like group because of the potential change in treatment recommendations and it would therefore have been preferential to have analyzed the SN ratio on a large enough luminal A-like group. The SN ratio predictions for the luminal A-like group was significant but we chose to include the other subtypes in our study as well in order to achieve a high enough power since we saw no reason for the SN predictions to vary depending on subtype.
Conclusion
Our results indicate that around one sixth of patients with a luminal A-like tumor may be undertreated with adjuvant chemotherapy due to a false low axillary staging, if the indication for ALND is set to >2 positive SNs. We hypothesize that many of these patients can be identified when two to three SNs can be analyzed as we found a significant relationship between the ratio of positive SNs compared to the total amount of examined SNs and the total number of LNMs.
Disclosure statement
No financial disclosures from any of the authors.
Additional information
Funding
References
- Dengel LT, Van Zee KJ, King TA, et al. Axillary dissection can be avoided in the majority of clinically node-negative patients undergoing breast-conserving therapy. Ann Surg Oncol. 2014;21:22–27.
- Curigliano G, Burstein HJ, P Winer E, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen international expert consensus conference on the primary therapy of early breast cancer 2017. Ann Oncol. 2017;28:1700–1712.
- Diessner J, Wischnewsky M, Blettner M, et al. Do patients with luminal A breast cancer profit from adjuvant systemic therapy? A retrospective multicenter study. PLoS One. 2016;11:e0168730.
- Nielsen TO, Jensen MB, Burugu S, et al. High-risk premenopausal luminal A breast cancer patients derive no benefit from adjuvant cyclophosphamide-based chemotherapy: results from the DBCG77B clinical trial. Clin Cancer Res. 2017;23:946–953.
- Uchida N, Suda T, Ishiguro K. Effect of chemotherapy for luminal a breast cancer. Yonago Acta Med. 2013;56:51–56.
- Fisher B, Jeong JH, Bryant J, et al. Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from national surgical adjuvant breast and bowel project randomised clinical trials. Lancet. 2004;364:858–868.
- Goldhirsch A, Wood WC, Gelber RD, et al. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18:1133–1144.
- International Breast Cancer Study, G., Endocrine responsiveness and tailoring adjuvant therapy for postmenopausal lymph node-negative breast cancer: a randomized trial. J Natl Cancer Inst. 2002;94:1054–1065.
- Noda S, Onoda N, Asano Y, et al. Predictive factors for the occurrence of four or more axillary lymph node metastases in ER-positive and HER2-negative breast cancer patients with positive sentinel node: a retrospective cohort study. Int J Surg. 2016;26:1–5.
- Fisher B, Bauer M, Wickerham DL, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer. 1983;52:1551–1557.
- Jatoi I, Hilsenbeck SG, Clark GM, et al. Significance of axillary lymph node metastasis in primary breast cancer. J Clin Oncol. 1999;17:2334–2340.
- Albain KS, Barlow WE, Ravdin PM, et al. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:2055–2063.
- Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65.
- Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717.
- Early Breast Cancer Trialists' Collaborative Group; Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444.
- Goldvaser H, Majeed H, Ribnikar D, et al. Influence of control group therapy on the benefit from dose-dense chemotherapy in early breast cancer: a systemic review and meta-analysis. Breast Cancer Res Treat. 2018;169(3):413–425.
- Gnant M, Thomssen C, Harbeck N. St. Gallen/Vienna 2015: a brief summary of the consensus discussion. Breast Care (Basel). 2015;10:124–130.
- Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206–2223.
- Maisonneuve P, Disalvatore D, Rotmensz N, et al. Proposed new clinicopathological surrogate definitions of luminal A and luminal B (HER2-negative) intrinsic breast cancer subtypes. Breast Cancer Res. 2014;16:R65.
- Ehinger A, Malmstrom P, Bendahl PO, et al. Histological grade provides significant prognostic information in addition to breast cancer subtypes defined according to St Gallen 2013. Acta Oncol. 2017;56:68–74.
- Regionala cancercentrum i samverkan. Swedish National Guidelines for Breast cancer. 2018 [cited 2018 May]; Available at: https://www.cancercentrum.se/samverkan/cancerdiagnoser/brost/vardprogram/gallande-vardprogram/
- Nieweg OE, Jansen L, Valdes Olmos RA, et al. Lymphatic mapping and sentinel lymph node biopsy in breast cancer. Eur J Nucl Med. 1999;26:S11–S16.
- Pesek S, Ashikaga T, Krag LE, et al. The false-negative rate of sentinel node biopsy in patients with breast cancer: A meta-analysis. World J Surg. 2012;36:2239–2251.
- van der Ploeg IM, Nieweg OE, van Rijk MC, et al. Axillary recurrence after a tumour-negative sentinel node biopsy in breast cancer patients: a systematic review and meta-analysis of the literature. Eur J Surg Oncol. 2008;34:1277–1284.
- Degnim AC, Griffith KA, Sabel MS, et al. Clinicopathologic features of metastasis in nonsentinel lymph nodes of breast carcinoma patients. Cancer. 2003;98:2307–2315.
- Durak MG, Akansu B, Akin MM, et al. Factors predicting non-sentinel lymph node involvement in sentinel node positive breast carcinoma. Turk Patoloji Derg. 2011;27:189–195.
- Ozmen V, Karanlik H, Cabioglu N, et al. Factors predicting the sentinel and non-sentinel lymph node metastases in breast cancer. Breast Cancer Res Treat. 2006;95:1–6.
- Goyal A, Douglas-Jones A, Newcombe RG, et al. Predictors of non-sentinel lymph node metastasis in breast cancer patients. Eur J Cancer. 2004;40:1731–1737.
- Hung WK, Chan MC, Mak KL, et al. Non-sentinel lymph node metastases in breast cancer patients with metastatic sentinel nodes. ANZ J Surg. 2005;75:27–31.
- Ban EJ, Lee JS, Koo JS, et al. How many sentinel lymph nodes are enough for accurate axillary staging in t1-2 breast cancer? J Breast Cancer. 2011;14:296–300.
- Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111–121.