Abstract
Advances in colorectal polyp detection and resection methods aim to reduce interval cancer rates. Complete polypectomy is essential to reduce the risk of early recurrence and the development of interval cancers. To achieve this, polyps must first be correctly identified and then completely excised. This article reviews current adenoma detection methods in use and the management of residual disease.
Introduction
The identification and removal of colonic polyps reduces the incidence and mortality from colorectal cancer and forms the basis for all colorectal cancer screening programs [Citation1]. Despite the success of colorectal cancer screening programs interval cancers do occur. Missed lesions at the time of index colonoscopy and inadequate excision account for a significant proportion of interval cancers. Numerous practice recommendations and guidelines are available which endeavor to improve adenoma detection rates (ADR). ADRs are pivotal key performance indices for colonoscopy quality assessment, with individual ADRs often seen as a surrogate marker of overall colonoscopy quality.
Polyp detection is only one part of a successful colorectal cancer prevention strategy. Performing safe and complete polypectomy is also a specific goal for successful colonoscopy. Better recognition and visualization of margins, improved excision techniques and identification and removal of residual polyp tissue have all been the subject of clinical investigation, with a view to reducing the risk of early recurrence and interval cancers. Inclusion of appropriate polypectomy technique in the latest ESGE position statement on performance measures for lower gastrointestinal endoscopy as one of seven key indices highlights the importance of complete excision, improvement in polypectomy technique and early detection and management of residual disease to avoid interval cancers [Citation2].
Interval cancer rates and etiology
Interval cancer refers to the occurrence of colorectal cancer following a colonoscopy prior to the next scheduled surveillance procedure. While some interval cancers represent rapid neoplastic development through alternate neoplastic pathways from the traditional, normal mucosa - polyp – dysplasia - cancer sequence, the majority (up to 70%) are thought to arise from “missed” or “incompletely excised” precancerous polyps at the time of index colonoscopy [Citation3]. The rate of interval cancers is therefore considered to reflect the general quality of colonoscopy within screening programs, at an institution and for individual Endoscopists. A meta –analysis from 2014 reported post colonoscopy interval cancers rates of 2–9% [Citation4]. More recent studies have reported lower interval cancer rates, 0.07%, or 1 in 1,400 colonoscopies, which may indicate improved colonoscopy quality overtime [Citation5,Citation6]. Older age, female gender, proximal polyp location and the presence of diverticulosis at the time of index colonoscopy have all been associated with increased interval cancer risk.
The majority, 80%, of post colonoscopy cancers are thought to result from missed polyps [Citation5]. There is significant evidence from “back to back” colonoscopy studies to support this suggestion with reported adenoma miss rates ranging from 7% employing newer technologies to 48% for standard colonoscopy [Citation7–10]. As a result, there is significant effort and energy being employed to enhance technology and improve individual practice to reduce adenoma miss rates.
Overcoming incomplete excision, which accounts for ∼20% of interval cancers, remains a challenge. Improved polypectomy techniques, with enhanced detection and management of residual disease, may increase the complete removal of precancerous lesions and is another important goal to avoid what are, by definition, preventable colorectal cancers.
Polypectomy technique recommendations
The European society of gastrointestinal endoscopy (ESGE) clinical guideline strongly recommends, with high quality evidence, that all polyps be resected, except for diminutive rectal and recto-sigmoid polyps that can be predicted with high confidence to be hyperplastic [Citation11]. The ESGE guideline also recommends resection using cold snare polypectomy (CSP) for adenomas <5 mm as CSP achieves high rates of complete resection safely. Similarly, CSP is the recommended polypectomy technique for lesions from 5 to 9 mm on the basis of a superior safety profile. Hot snare polypectomy (HSP) should be used to remove sessile lesions 10–19 mm in size and pedunculated lesions, with prior elevation to avoid cautery injury. Although there is evidence from a recent study to show that piece meal excision by CSP of larger sessile polyps is feasible, effective and safe and is likely to become a viable option going forward for larger lesions [Citation12].
Cold forceps polypectomy (CFP) has fallen out of favor even for smaller lesions as there is evidence to show higher incomplete resection rates with CFB versus CSP. In one prospective study of CFP with complete visual excision followed by EMR of the polypectomy site, subsequent histological examination showed residual disease in 61%. [Citation13]. Nonetheless, higher complete resection rates, up to 100% [Citation14] with CFP have been reported particularly for smaller lesions of 1–3 mm. The technique remains popular in clinical practice for diminutive polyps and can be considered for lesions 1–3 mm in size when CSP is difficult. Similarly, Hot Forceps Polypectomy (HFP) is associated with higher incomplete resection rates compared with snare polypectomy. HFP causes more complications, while also damaging tissue thereby negatively affecting subsequent histological examination and is no longer recommended for routine clinical use. Alternative resection methods are increasing in popularity, including “lift and cut” and suction pseudo polyp techniques, underwater and wide field EMR, as well as full thickness resection and endoscopic submucosal dissection (ESD). However, there is insufficient evidence currently available to support any one specific technique over another or for them to be routinely included in evidence-based guidelines. Future recommended resection methods will likely vary according to patient and polyp characteristics as Endoscopist experience and the body of evidence increases.
Residual adenoma/dysplastic tissue
To reduce the risk of polyp recurrence and therefore interval cancers, complete excision of identified lesions at the time of the index colonoscopy should be the treatment goal. Complete histological resection is defined as the absence of dysplastic tissue from both the vertical and horizontal resected polyp margins. While routine reporting of completeness of excision would be desirable, it is not always achievable. Histological reporting of complete excision is easier for en bloc resections and can be problematic for cold biopsy and piecemeal polypectomy specimens. A recent prospective study by Matsuura et al. [Citation15] reported that the lateral margins of sessile lesions (<10 mm) excised by CSP could not be adequately assessed in 67% of cases.
Reported R0 resection rates from a variety of settings are available in the literature. R0 resection rates using CSP for diminutive and small colorectal polyps range from 53 to 98% [Citation16–20]. R0 resection rates for alternative techniques, HSP and EMR are also available and are similar to CSP, likely reflecting appropriate selection of polypectomy technique based on polyp morphology [Citation19,Citation21] ().
Table 1. R0 resection rates by polypectomy technique and size.
Histological reporting of R0 resections is only one means of assessing residual disease. Other techniques include sampling the margin of a polypectomy using either a biopsy forceps or by EMR at the time of polypectomy or of the scar sites on follow up interval colonoscopy. Studies employing these techniques have often reported significant rates of residual dysplastic tissue.
A recent follow-up colonoscopy study of CSP resected lesions <9 mm in size from Japan, reported a very low residual disease rate of only 0.98% from biopsies of the scar. In this study, there was a 3-week interval between polypectomy and follow up colonoscopy and 92% of polypectomy scars were identified. It remains a possibility that mucosal healing may have obscured some residual disease, accounting for differences with EMR and cold forceps reported rates of residual disease [Citation22].
Several studies have reported incomplete resection rates (IRR) by assessing the proportion of polypectomy margins with residual dysplastic tissue, sampled by either biopsy or EMR at the time of index polypectomy [Citation23,Citation24]. A recent study by Kim et al. investigating the efficacy of HSP with or without epinephrine lift, reported residual disease in 12% of cases following HSP for sessile polyps 5–9 mm in size. While the rate of residual disease decreased with EMR to 7%, this did not reach statistical significance [Citation25]. Zang et al. also in 2018 reported incomplete resection rates for sessile polyps 6–9 mm of 8.5% and 1.5% for CSP and EMR, respectively [Citation26]. Lower CSP incomplete resection rates were shown by Dwyer et al. in their study to assess the efficacy of dedicated cold snares versus traditional snares. They reported complete resection rates of 98.4%, with dedicated cold snares also using biopsy sampling of polyp margins [Citation27]. Perhaps surprisingly similar incomplete resection rates have been reported for various polypectomy techniques used to remove diminutive lesions <5 mm, employing EMR sampling [Citation28,Citation29] .
Table 2. Incomplete resection rates by polypectomy technique and size.
Risk factors for incomplete polypectomy
Increasing polyp size is a recognized risk factor for incomplete resection. Pohl et al. in 2013 reported a relative risk of 2.1 of incomplete resection for polyps >10 mm. Overall the IRR reported in this study was 17% and 7% for polyps 10–20 mm and 5–9 mm, respectively [Citation30]. In a retrospective large single center study of 791 neoplastic lesions the authors reported an overall R0 resection rate of 71% and positive margins were significantly higher 41% vs 28%, (p = .007) in polyps ≥10 mm [Citation31]. While a prospective study designed to assess the efficacy of EMR compared to HSP for larger polyps (10–25mm) also found a significant difference in R0 resection rates based on size. A polyp size of >15 mm was four times more likely to be incompletely resected. R0 resections by EMR were reported in 90% of polyps <19 mm versus 75% for those 20 mm and larger [Citation21].
Several of these studies have also found sessile serrated polyp (SSP) compared to standard adenoma histology to be a risk factor for residual disease as assessed by margin EMR and biopsy, with odds ratios of 2.8 and 3.4, respectively [Citation25,Citation30,Citation31]. Similarly, one retrospective study of large >10 mm, SSPs (N = 101) and adenomas (N = 192) confirmed higher rates of residual disease on histology after en bloc EMR resection for SSPs compared to adenomas, 45% versus 38%. Interestingly, they also reported a high rate, 6.5% (n = 10) of residual disease at resection sites on 153 follow-up colonoscopies a mean of 13 months after index polypectomy. In addition, residual neoplasia was noted in 3/68 lesions (4.4%) with negative margins originally compared to 5/60 lesions (8.3%) with positive original margins (p = .47), highlighting the need for vigilance even for reported R0 resections [Citation32].
Piecemeal resections are associated with an increased risk of local recurrence and the risk increases with the number of pieces removed [Citation33,Citation34]. Piecemeal resection also interferes with histological assessment, by interrupting the polyp margin unless the lesion is pinned in position prior to assessment, which is infrequently performed.
Currently, both the American society for gastrointestinal endoscopy (ASGE) and the European society for gastrointestinal endoscopy (ESGE) advise performing a follow-up endoscopy within 3–6 months after piecemeal resection.
Residual adenoma/dysplastic tissue detection
Although early reinspection of at-risk cases and detection of residual or recurrent disease has a key role in minimizing interval cancer rates, appropriate identification and removal of residual dysplastic tissue at the time of polypectomy should be of paramount importance. Identification at the time of polypectomy would seem preferable to detection of recurrence on follow up colonoscopy at some later stage, as missed residual disease has the potential to become advanced in the interval.
Standard inspection with white light, while important, is not without its limitations. Indeed, with the rise in CSP popularity, examination for residual disease can be complicated by the frequent occurrence of defect protrusions. One study of 257 CSPs reported protruding residual tissue in 14% of CSP defects. In all cases, further histological assessment confirmed the absence of residual dysplastic tissue [Citation35]. However, at the time of polypectomy and without histology this may appear less clear-cut.
While it is now recommended and commonplace to use augmented imaging techniques to identify and characterize lesions prepolypectomy, including high definition white light and magnification endoscopy, narrow band imaging (NBI), Flexible spectral imaging color enhancement (FICE), and chromo-endoscopy [Citation11]. There has been less focus on their potential role in identifying incomplete excision and residual dysplastic tissue at the time of polypectomy or recurrent disease on follow up. There is some evidence to support augmented imaging techniques. One recent study used NBI to help determine complete excision of diminutive polyps (<5 mm) being removed by CFP compared to standard CSP technique. They found similar overall complete resection rates for the two techniques, NBI-CFP 91% and CSP 93%. Not unexpectedly for polyps >3 mm CSP was more effective than NBI-CFP, (93% vs. 87% complete resection), however it remains possible that NBI augmented CSP would have yielded superior complete resection rates also [Citation36].
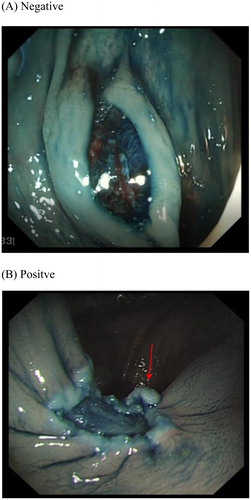
Our own study using post polypectomy and EMR chromo-endoscopy with 0.13% Indigo Carmine showed a significant rise in residual disease detection compared to close inspection alone with standard while light endoscopy. In our cohort of predominantly larger polyps (>5 mm), residual disease was identified in 19.5% post polypectomy by margin biopsy. White light endoscopy () accurately detected residual disease in 55%, compared to 76.5% with indigo carmine chromo endoscopy (). The overall missed residual disease rate was thereby reduced to 4.6% (p = .03) with chromo endoscopy post polypectomy [Citation37]. Augmented imaging techniques may also enhance detection of residual and/or recurrent disease at the site of previous resection. One recent study from Australia in 2017 found High Definition White Light (HD-WL) combined with NBI enhanced endoscopic detection of recurrence when examining previous EMR scars. Of 183 scars histology confirmed recurrence in 16% (n = 30) while augmented imaging, predicted recurrence in 20% (n = 37). The NPV for augmented imaging was high 98% [Citation38], suggesting a negative examination with HD-WL and NBI accurately out rules recurrence.
Figure 1. (A) Negative and (B) Positive post snare polypectomy margin and base as viewed with standard white light.
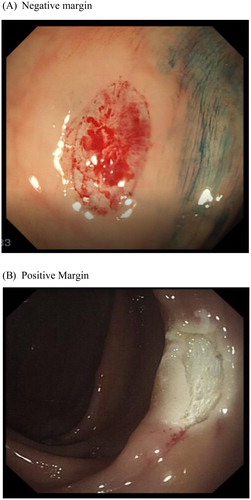
Figure 2. (A) Negative and (B) Positive post snare polypectomy margin and base as viewed following application of 0.13% Indigo Carmine.
Computer assisted detection programs are being increasingly evaluated as a method to enhance real time polyp detection. One recent study proposes a new polyp detection method based on a three-way image presenter and convolutional neural networks. This system is taught a variety of polyp features such as color, texture, shape, and temporal information in multiple scales, thereby enabling a more accurate polyp localization during colonoscopy [Citation39].
Residual adenoma/dysplastic tissue management
Unlike residual disease detected on surveillance colonoscopy, for which the ESGE recommends snare-resection within the same procedure, there are no clear recommendations for managing residual dysplastic tissue at the time of index polypectomy. Although the same techniques are likely to prove beneficial.
As for index polypectomy, in most cases cold snare resection is an effective means of removing residual disease. Snare polypectomy of residual disease was successful in 135 of 145 (93%) cases of early recurrence in a large follow up study of 799 patients post EMR [Citation33]. Of interest in this study, use of adjunctive Argon Plasma Coagulation (APC) during the initial EMR was a risk factor for early recurrence (OR 3.5). Suggesting there was recognition and management of incomplete resection at the time of index EMR with APC, which was ineffective. Similarly, results of a paper by Regula et al. on the application of adjunctive APC to visible residual disease either at index colonoscopy or on follow up were disappointing. After piece meal snare resection of 82 sessile polyps APC was applied in 63 cases. APC had no effect on complete resection rates, 100% versus 90%, or subsequent adenoma recurrence rate, 14% for both groups [Citation40]. Although there is contradictory, evidence available from earlier studies in support of the use of APC as an adjunct during piece meal polypectomy [Citation41–43]. Also in their discussion, Regula et al. also suggest APC is ultimately effective if a repeated surveillance (every 3 months in the first year) and treatment regimen is employed, reporting 100% success in appropriate cases.
EMR has proven superior to a variety of modalities to destroy residual polyp tissue, although not all scar tissue or residual polyp will lift [Citation33]. Hot avulsion is a popular technique that has been applied to small areas of nonlifting residual or recurrent polyp and has been reported to be effective in small prospective studies [Citation44,Citation45]. Although, as with APC repeated treatments are likely to be required to be fully effective. Additional strategies being explored for non-lifting tissue including cold avulsion in conjunction with thermal ablation, ESD and Underwater EMR with varying degrees of success [Citation46,Citation47]. Other previously explored modalities including hot snare excision, laser ablation and delayed avulsion have all fallen out of favor.
Conclusion
Incomplete polypectomy is thought to account for up to 20% of preventable interval colorectal cancers. Selection of appropriate polypectomy techniques can safely improve complete resection rates and international recommendations should be adhered to. Improving awareness among Endoscopists of the significant risk of incomplete resection is an important step toward improving polypectomy standards. Unlike adenoma detection rates and other quality measures which all aim to improve lesion detection, there has traditionally been less focus on completeness of excision.
Inclusion of adherence with polypectomy technique in colonoscopy quality measures should be included in all routine endoscopy audits. In addition, Endoscopists’ should keep abreast of the current literature with a view to adopting proven novel resection technologies as they become available.
Close inspection of all polypectomy margins should be undertaken at the time of excision, with application of augmented techniques employed to enhance lesion identification and characterization. Many of these techniques are already commonly employed in routine colonoscopy for other indications and are therefore familiar to the Endscopist. Identified residual polyp tissue should be excised by either snare excision or EMR where possible. If adjunctive APC or hot avulsion is employed, early follow up colonoscopy at 3 months is likely to be required to treat additional disease and/or early recurrence. Marking of lesions to assist with scar identification is advisable when follow up colonoscopies and additional therapy are likely.
Additional improvement in resection techniques and management of incomplete resections is required to improve patient outcomes and warrants additional research focus and energy.
Disclosure statement
The authors report no conflicts of interest.
References
- Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696.
- Kaminski MF, Thomas-Gibson S, Bugajski M, et al. Performance measures for lower gastrointestinal endoscopy: A European society of gastrointestinal endoscopy (ESGE) quality improvement initiative. Endoscopy. 2017;49:378–397.
- Robertson DJ, Lieberman DA, Winawer SJ, et al. Colorectal cancers soon after colonoscopy: A pooled multicohort analysis. Gut. 2014;63:949–956.
- Singh S, Singh PP, Murad MH, et al. Prevalence, risk factors, and outcomes of interval colorectal cancers: A systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1375–1389.
- Iwatate M, Kitagawa T, Katayama Y, et al. Post-colonoscopy colorectal cancer rate in the era of high-definition colonoscopy. World J Gastroenterol. 2017;23:7609–7761.
- Humphrey F, Gastañaduy M, Smith J, et al. Interval colorectal cancers at ochsner medical center: Where do we stand. Ochsner J. 2017;17:322–327. Winter
- Gralnek IM, Siersema PD, Halpern Z, et al. Standard forward viewing colonoscopy versus full-spectrum endoscopy: An international, multicentre, randomised, tandem colonoscopy trial. Lancet Oncol. 2014;15:353–360.
- Dik VK, Gralnek IM, Segol O, et al. Multicenter, randomized, tandem evaluation of EndoRings colonoscopy-results of the CLEVER study. Endoscopy. 2015;47:1151–1158.
- Halpern Z, Gross SA, Gralnek IM, et al. Comparison of adenoma detection and miss rates between a novel balloon colonoscope and standard colonoscopy: A randomized tandem study. Endoscopy. 2015;47:238–244.
- Pioche M, Denis A, Allescher HD, et al. Impact of 2 generational improvements in colonoscopes on adenoma miss rates: Results of a prospective randomized multicenter tandem study. Gastrointest Endosc. 2018;88(1):107–116.
- Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European society of gastrointestinal endoscopy (ESGE) clinical guideline. Endoscopy. 2017;49:270–229.
- Tate DJ, Awadie H, Bahin FF, et al. Wide-field piecemeal cold snare polypectomy of large sessile serrated polyps without a submucosal injection is safe. Endoscopy. 2017;50:248–252.
- Efthymiou M, Taylor AC, Desmond PV, et al. Biopsy forceps is inadequate for the resection of diminutive polyps. Endoscopy. 2011;43:312–316.
- Jung YS, Park JH, Kim HJ, et al. Complete biopsy resection of diminutive polyps. Endoscopy. 2013;45:1024–1102.
- Matsuura N, Takeuchi Y, Yamashina T, et al. Incomplete resection rate of cold snare polypectomy: a prospective single-arm observational study. Endoscopy. 2017;49:251–225.
- Kim JS, Lee BI, Choi H, et al. Cold snare polypectomy versus cold forceps polypectomy for diminutive and small colorectal polyps: A randomized controlled trial. Gastrointest Endosc. 2015;81:741–747.
- Din S, Ball AJ, Riley SA, et al. A randomized comparison of cold snare polypectomy versus a suction pseudopolyp technique. Endoscopy. 2015;47:1005–1101.
- Horiuchi A, Hosoi K, Kajiyama M, et al. Prospective, randomized comparison of 2 methods of cold snare polypectomy for small colorectal polyps. Gastrointest Endosc. 2015;82:686–669.
- Noda H, Ogasawara N, Sugiyama T, et al. The influence of snare size on the utility and safety of cold snare polypectomy for the removal of colonic polyps in Japanese patients. J Clin Med Res. 2016;8:662–666.
- Aslan F, Camcı M, Alper E, et al. Cold snare polypectomy versus hot snare polypectomy in endoscopic treatment of small polyps. Turk J Gastroenterol. 2014;25:279–283.
- Horiuchi A, Makino T, Kajiyama M, et al. Comparison between endoscopic mucosal resection and hot snare resection of large nonpedunculated colorectal polyps: a randomized trial. Endoscopy 2016; 48:646–651.
- Maruoka D, Arai M, Akizue N, et al. Residual adenoma after cold snare polypectomy for small colorectal adenomas: A prospective clinical study. Endoscopy. 2018;50(7):693–700.
- Kawamura T, Takeuchi Y, Asai S, et al. A comparison of the resection rate for cold and hot snare polypectomy for 4-9 mm colorectal polyps: a multicentre randomised controlled trial (CRESCENT study). Gut. 2017;67(11):1950–1957.
- Papastergiou V, Paraskeva KD, Fragaki M, et al. Cold versus hot endoscopic mucosal resection for nonpedunculated colorectal polyps sized 6–10 mm: A randomized trial. Endoscopy. 2018;50:403–411.
- Kim HS, Jung HY, Park HJ, et al. Hot snare polypectomy with or without saline solution/epinephrine lift for the complete resection of small colorectal polyp. Gastrointest Endosc. 2018;87(6):1539–1547.
- Zhang Q, Gao P, Han B, et al. Polypectomy for complete endoscopic resection of small colorectal polyps. Gastrointest Endosc. 2018;87:733–737.
- Dwyer JP, Tan JYC, Urquhart P, et al. A prospective comparison of cold snare polypectomy using traditional or dedicated cold snares for the resection of small sessile colorectal polyps. Endosc Int Open. 2017;5:E1062–E1068.
- Gómez V, Badillo RJ, Crook JE, et al. Diminutive colorectal polyp resection comparing hot and cold snare and cold biopsy forceps polypectomy. Results of a pilot randomized, single-center study (with videos). Endosc Int Open. 2015;3:E76–E80.
- Lee CK, Shim JJ, Jang JY. Cold snare polypectomy vs. cold forceps polypectomy using double-biopsy technique for removal of diminutive colorectal polyps: A prospective randomized study. Am J Gastroenterol. 2013;108:1593–1600.
- Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74–80.
- Hirose R, Yoshida N, Murakami T, et al. Histopathological analysis of cold snare polypectomy and its indication for colorectal polyps 10–14 mm in diameter. Dig Endosc. 2017;29:594–560.
- Agarwal A, Garimall S, Scott FI, et al. En bloc endoscopic mucosal resection is equally effective for sessile serrated polyps and conventional adenoma. Surg Endosc. 2018;32:1871–1878.
- Moss A, Williams SJ, Hourigan LF, et al. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: Results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut. 2015;64:57–65.
- Oka S, Tanaka S, Saito Y, et al. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol. 2015;110:697–670.
- Tutticci N, Burgess NG2, Pellise M, et al. Characterization and significance of protrusions in the mucosal defect after cold snare polypectomy. Gastrointest Endosc. 2015;82:523–528. Epub 2015 Apr 22.
- Park SK, Ko BM, Han JP, et al. A prospective randomized comparative study of cold forceps polypectomy by using narrow-band imaging endoscopy versus cold snare polypectomy in patients with diminutive colorectal polyps. Gastrointest Endosc. 2016;83:527–532.
- O’Morain N, Connolly E, Shahin A, et al. Chromoendoscopy post-EMR improves detection of incomplete polyp resection. Mo 1690 DDW Abstract #2910972. Gastrointerest Endosc. 2018;87:Supplement Issue 6 Page AB480.
- Desomer L, Tutticci N, Tate DJ, et al. A standardized imaging protocol is accurate in detecting recurrence after EMR. Gastrointest Endosc. 2017;85:518–526.
- Tajbakhsh N, Gurudu SR, Liang J. Automatic polyp detection in colonoscopy videos using an ensemble of convolutional neural networks. Proceedings International Symposium on Biomedical. Imaging12th IEEE International Symposium on Biomedical Imaging, ISBI 2015; Brooklyn, United States.
- Regula J, Wronska E, Polkowski M, et al. Argon plasma coagulation after piecemeal polypectomy of sessile colorectal adenomas: Long-term follow-up study. Endoscopy. 2003;35(3):212–218.
- Zlatanic J, Waye JD, Kim PS, et al. Large sessile colonic adenomas: Use of argon plasma coagulator to supplement piecemeal snare polypectomy. Gastrointest Endosc. 1999;49:731–735.
- Grund KE, Storek D, Farin G. Endoscopic argon plasma coagulation (APC): first clinical experiences in flexible endoscopy. Endosc Surg Allied Technol. 1994;2:42–46.
- Neneman B, Gasiorowska A, Malecka-Panas E. The efficacy and safety of argon plasma coagulation (APC) in the management of polyp remnants in stomach and colon. Adv Med Sci. 2006;51:88.
- Andrawes S, Haber G. Avulsion: a novel technique to achieve complete resection of difficult colon polyps. Gastrointest Endosc. 2014;80:167.
- Veerappan SG, Ormonde D, Yusoff IF, et al. Hot avulsion: a modification of an existing technique for management of non-lifting areas of a polyp (with video). Gastrointest Endosc. 2014;80:884–888.
- Sakamoto T, Saito Y, Matsuda T, et al. Treatment strategy for recurrent or residual colorectal tumors after endoscopic resection. Surg Endosc. 2011;25:255–260.
- Kim HG, Thosani N, Banerjee S, et al. Underwater endoscopic mucosal resection for recurrences after previous piecemeal resection of colorectal polyps (with video). Gastrointest Endosc. 2014;80:1094–1110.
