Abstract
Background: Patients with a BRCA1 or BRCA2 mutation (BRCA-mutated breast cancer) are frequently diagnosed with low differentiated and highly proliferating breast cancer characterized by high amounts of tumor-infiltrating lymphocytes (Tils). Stromal Tils (sTils) are highly prognostic in sporadic triple-negative and HER2 positive breast cancer however, their prognostic importance in BRCA-mutated breast cancers is unknown.
Material and methods: Formalin-fixed paraffin-embedded primary tumor tissue from 411 patients with a germline BRCA1 or BRCA2 mutation and diagnosed with early breast cancer was included. The percentage of sTils was quantified on full HE sections according to guidelines proposed by the Immuno-Oncology Biomarker in Breast Cancer Working Group. Distribution of sTils and associates with patient and tumor characteristics were assessed according to categorical sTils groups defined as low (<10%), intermediate (10–59%) and high (≥60%). Prognostic associations of sTils were evaluated as a continuous variable in univariate and multivariate models. Only follow-up time beyond date of BRCA mutation test was included.
Results: A large proportion had high sTils (27% in the full cohort, 36% in BRCA1-mutated, and 44% in ER negative breast cancers). Higher sTils were associated with BRCA1, ER negative breast cancer, high histological grade and medullary histology. In combined analysis for BRCA1 and BRCA2-mutated breast cancers, increasing sTils in 10% intervals were significantly associated with OS (HR 0.92, 95% CI 0.84–1.00, p = .05). For each 10% increment of sTils in BRCA1 breast cancers, a 10% reduction of mortality (adjusted HR 0.90 95% CI 0.81–0.99, p = .03) and a 13% reduction in risk of DFS-event (HR 0.87 95% CI 0.76–1.00, p = .05) was observed even after adjustment for ER status. No significant association with survival was of observed in the BRCA2 subgroup. Test for interaction of sTils and BRCA status was not statistically significant (p = .3).
Conclusions: Breast cancer patients with a germline BRCA mutation had higher sTils than previously reported in sporadic breast cancers, and sTils were associated with favorable survival among BRCA carriers.
Introduction
Breast cancers in patients with a germline BRCA1 mutation (BRCA1-mutated breast cancer) often present with a prominent immune infiltrate in the tumor-micro-environment indicating that these tumors are highly immunogenic [Citation1]. BRCA1-mutated breast cancers are molecularly associated with increased genomic instability [Citation2,Citation3] manifesting in high proliferative index and low differentiation, and consequently high histological grade [Citation1,Citation4–7]. Most BRCA1-mutated tumors are additionally triple-negative (TN), and are associated with a worse prognosis in part due to lack of targetable therapies for this subtype. BRCA2 mutated breast cancers are mainly estrogen receptor (ER) positive and high-grade [Citation4].
Increasing sTils levels reported in 10% intervals have repeatedly been associated with reduction of recurrence and death by around 15–20% [Citation8–12], however only in TN/basal-like and HER2 positive subtype. In ER positive breast cancers, sTils have been associated with improved survival in high-grade tumors only: they may even have a worse prognostic impact in grade 1 and 2 tumors [Citation13]. Certain chemotherapeutics (e.g., anthracyclines) may modulate the immune response facilitated by so-called ‘immunogenic cell death’ [Citation14,Citation15]. The mechanism behind this is the release of an abundance of peptides forming multiple cancer- specific antigens which activate the immune system. High cancer antigen presentation is in part associated with high mutational burden due to mutational altered gene products [Citation16]. This cancer antigen formation is high in tumors with DNA-repair defects. Accordingly, hereditary cancers with deficient DNA-repair manifest with high mutational rate and high lymphocytic infiltration [Citation16,Citation17].
However, the interaction of the immune system with cancer development and progression is complex and affected by individual genetic, environmental and tumor-specific factors [Citation16]. In addition, it is dynamic and depends on inhibitory and activating signals forming an either pro- or anti-tumorigenic milieu reflected in different compositions of immune cells and expression of inhibitory pathways (e.g., PD-1 and CTLA4 amongst others) [Citation18]. Modulation of the immune response, for instance, with check-point inhibitors have proven effective in highly mutated/genomic instable tumors [Citation16].
In light of the germline mutations in the BRCA1 and BRCA2 gene associated with deficient homologous repair (HR) and high immune response, BRCA-mutated breast cancers may constitute a breast cancer subtype in which immune modulation by, for instance, check-point inhibitors are beneficial. However, characterization and quantification of Tils have not previously been described using a standardized method in a larger cohort of germline BRCA-mutated breast cancers. The guidelines published by the International Immuno-Oncology Biomarker in Breast Cancer Working Group (IOBWG) serve as a standardized and accepted assessment method for sTils quantification in breast cancer [Citation19]. Consequently, this study investigated sTils in a nationwide Danish cohort of BRCA1/2 carriers with primary breast cancer in terms of prevalence in sTils groups (low, intermediate and high), distributional differences according to BRCA gene, and prognostic impact by each 10% sTils increment.
Material and methods
The National Committee on Health Research Ethics (registration number 33483) and the Danish Data Protection Agency (2009-41-3611) approved the study.
Patients
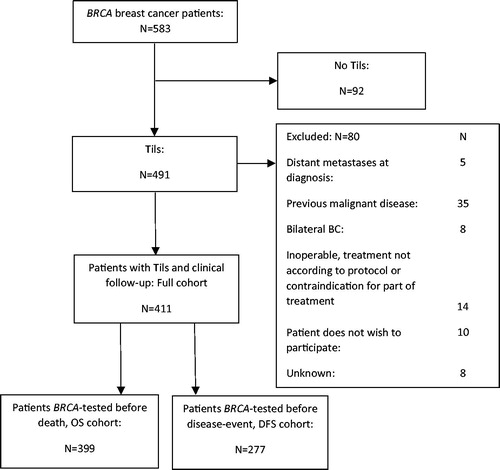
Since 1977, Danish women with primary breast cancer have been registered in the Danish Breast Cancer Cooperative Group (DBCG) database including prospectively registered clinical data. Family history of breast and ovarian cancer was added to the Hereditary Breast and Ovarian Cancer (HBOC) register in 1999 [Citation20]. From the DBCG database, Danish female BRCA1/2 carriers of BRCA mutation tested between 1997 and July 2011, with primary stage I–III breast cancer diagnosed between 1977 and February 2012, were included. Five-hundred and eighty-three breast cancer patients with BRCA1 or BRCA2 germline mutations were identified. Recently, survival data on patients from the same population diagnosed with a BRCA mutation less than two years after breast cancer surgery have been published [Citation21]. In the present study, all patients with sTils assessment and clinical follow-up (full cohort, n = 411) were included. Patients who had follow-up time exceeding BRCA test date contributed to survival analysis ().
BRCA mutation testing method, breast cancer treatment, clinical follow-up and medical registries were previously described [Citation21]. In brief, patients had clinical follow-up every third month the first year after breast cancer surgery, every sixth month during the following two to five years and once a year 6–10 years after breast cancer surgery or until a first event. Since 1997, patients suspected of having a germline BRCA1/2 mutations were referred for genetic counseling and, after risk stratification, offered mutational screening or predictive testing for BRCA1 and BRCA2 mutations. Proposed guideline criteria for referral to genetic counseling in 2001 [Citation22] were any of the following: young age at breast cancer diagnosis, first and second degree relatives with breast cancer or ovarian cancer, a disease-causing mutation in the family, breast and ovarian cancer in the same individual, and male breast cancer. However, to some extent, patients were referred by other criteria such as tumor biology or bilateral breast cancer. Clinical information was retrieved from the Danish National Patients Register (DNPR) [Citation23], the Danish Pathology Register (DPR) [Citation24] and the Danish Cancer Register [Citation25] as previously described [Citation21]. Mutational screening and predictive testing of the BRCA1 and BRCA2 genes for deleterious mutations and variant classification were performed as previously described [Citation21,Citation26,Citation27].
Tissue samples
Formalin-fixed paraffin-embedded (FFPE) tissue blocks were collected from Danish departments of pathology. Four-micrometer sections from each block were stained with hematoxylin and eosin (HE) for identification of tumor areas for preparation of tissue micro array (TMA) and for histological review. Up to four 1.5-micrometer cores from primarily the invasive tumor front were used for TMA. Review of histological subtype and grade was performed according to WHO guidelines [Citation22,Citation28,Citation29]. The diagnosis of ductal carcinoma with medullary characteristics was designated for high grade tumors with pushing margins and syncytial growth pattern in over 75% of the tumor in association with a pronounced lymphoplasmacytic infiltrate [Citation28].
IHC/ISH analysis
Immunohistochemical analysis was performed for ER, progesterone receptor (PR) and HER2 with anti-ER SP1 ready-to-use (RTU), anti-PR clone 1E2 RTU, and anti-HER2 clone 4B5 RTU (Ventana/Roche, USA). ER and PR were assessed as positive if ≥1% positive nuclear staining [Citation30], and HER2 IHC was scored according to standardized guidelines [Citation22]. For HER2 score 2+, HER2 gene status was determined with a gene-protein assay (INFORM Dual ISH DNA Probe cocktail, Ventana) and scored according to guidelines [Citation22,Citation31].
Tils evaluation
STils were assessed on full sections by light microscopy according to published guidelines by IOBWG for evaluation of sTils in breast cancer [Citation19]. Amount of sTils was assessed as percentage of the stromal area within the tumor bed occupied by mononuclear immune cells. Necrotic areas, intraepithelial lymphocytes, areas with intraductal carcinoma, and areas outside the boundaries of the tumor bed including a peripheral circumferential infiltrate were not included.
Statistical analysis
Both in the full cohort and within subgroups defined by BRCA gene and ER status, a Chi square trend test with two-sided p-value was used to test if patient and tumor characteristics were associated with increasing or decreasing sTils. Stromal Tils groups were defined as <10% sTils (low), 10–59% sTils (intermediate) and ≥60% sTils (high). Disease-free survival (DFS) was defined as time from definitive breast cancer surgery to local or distant relapse, contralateral breast cancer, death as first event, new primary cancer other than breast cancer, or end of clinical follow-up – whichever occurred first. Overall survival (OS) was defined as time to death of any cause. For OS, information about vital status was obtained by linkage to the Danish Civil Registration System, which ensures no loss of follow-up. To minimize longevity bias, data was left-truncated at time of BRCA-test for both DFS and OS. Consequently, only follow-up beyond date of BRCA test was included in analysis, with 399 patients with contribution of follow-up for OS and 277 for DFS. Median potential follow-up time from date of surgery and date of BRCA test were calculated using the Kaplan–Meier method. For OS, no patients were lost to follow-up and patients were only censored if alive at date of data retrieval (23 September 2016). For nine patients with missing BRCA test date, the date was set to 01 January 1999 if definitive surgery was performed before that date and otherwise was set to the date of definitive surgery. HRs associated with increasing sTils (continuous) were evaluated using a Cox model adjusted for ER status, lymph node status (positive/negative), histological grade and affected BRCA gene. Compliance with the proportional hazard assumption was tested by correlation of Schoenfeld residuals with ranked failure times and Schoenfeld residuals plotted against time. Consequently, ER status was included as a time-dependent variable and histological grade as strata. A significance level of 0.05 was chosen for two-sided p-values.
Results
STils groups
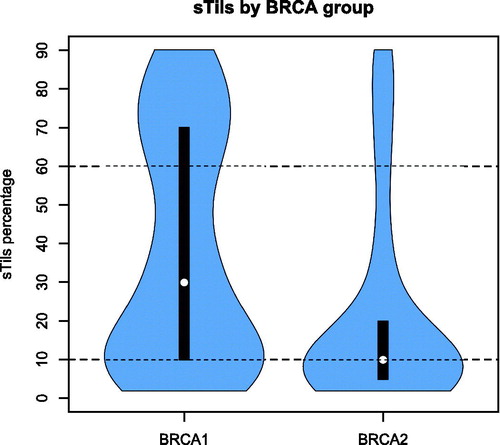
Of 583 BRCA-mutated breast cancers, 411 had results for sTils and clinical follow-up ( and ) of which 243 (59%) were BRCA1-mutated and 168 (41%) BRCA2-mutated. High, intermediate and low sTils were observed in 27%, 48% and 25% of tumors, respectively.
Table 1. Clinico-pathological characteristics and treatment according to sTils group in the full cohort.
The distribution of sTils varied according to affected BRCA gene, p < .0001 ( and ). The prevalence of high sTils was 27% in the full cohort, 36% in BRCA1-mutated and 44% in ER negative breast cancers. Prevalence of sTils groups and associates with clinic-pathological characteristics within the ER+/ER − and BRCA1/BRCA2 breast cancers are listed in Supplementary table A (see Supplementary Material). BRCA1 ER negative breast cancers had a high sTils percentage of 44% compared to 40% of BRCA2 ER negative tumors. In addition, among ER positive breast cancers, a higher proportion of BRCA1-mutated than BRCA2-mutated tumors were observed with high sTils (p = 0.02).
Irrespective of affected BRCA gene or ER status, increasing histological grade was associated with increasing sTils. Association with medullary histologically was observed in both BRCA1 and BRCA2, and in ER positive and ER negative breast cancers. Stromal Tils were not associated with breast cancer onset, tumor size or number of positive lymph nodes.
Stromal Tils and survival
Twelve patients died before BRCA test, and by left truncation 399 (97%) patients contributed with follow-up for OS (OS cohort). Similar, 277 (67%) were included with follow-up for DFS (DFS cohort), truncating patients with no clinical follow-up beyond BRCA test date. Similar proportions with low, intermediate and high sTils were observed in the OS cohort and DFS-cohort ().
Table 2. Percentage of BRCA1 and BRCA2 breast cancers with low, intermediate and high sTils percentage.
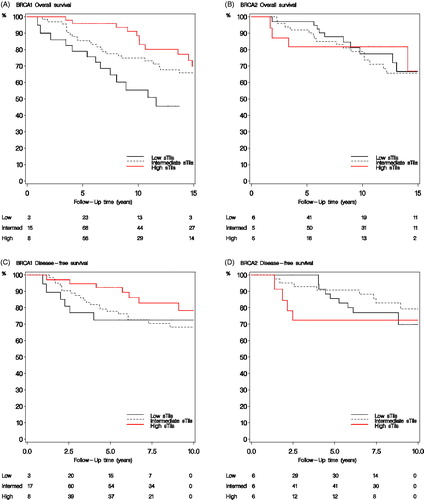
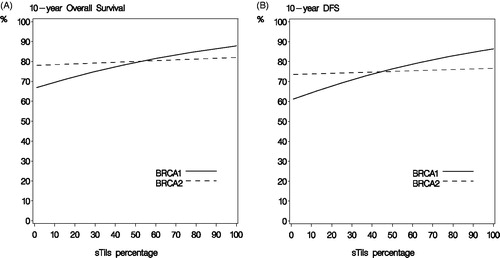
Median potential follow-up from breast cancer surgery for DFS and OS was 8.6 and 12.7 years, respectively. Median potential follow-up from BRCA test was 5.8 and 9.6 years, respectively. In multivariate analysis combined for BRCA1 and BRCA2-mutated breast cancers, increasing sTils in 10% intervals was associated with a lower mortality (HR 0.92, 95% CI 0.84–1.00, p = .05) and risk of DFS-event (HR 0.90, 95% CI 0.80–1.00, p = .06). In BRCA1-mutated breast cancers, high sTils were associated with longer survival () and similarly increasing sTils (continuous) showed both longer OS and DFS (). Accordingly, a BRCA1 carrier with 10% sTils would have a 10-year OS of 70% compared to 85% with 80% sTils. This translates into adjusted HR for OS of 0.90 (95% CI 0.81–0.99), p = .03 and for DFS of 0.87 (95% CI 0.76–1.00), p = .05 for each 10% increase in sTils. No association of sTils with prognosis was observed among BRCA2-mutated breast cancers () (adjusted HR for OS 0.98 (95% CI 0.85–1.13), p = 1.0 and for DFS 0.95 (0.79–1.15), p = .6) and, in addition, there was no difference in survival between sTils groups (). However, test for interaction of increasing sTils (continuous) and BRCA status was not significant (p = .3).
Discussion
Results show that the immune system is highly active in BRCA-mutated breast cancers, and increasing sTils are associated with a favorable outcome. Eight and 10% reduction of mortality and DFS-event, respectively, were observed for each 10% increase in sTils. As expected, this confirms that sTils are an important prognostic factor in BRCA breast cancers, and that increasing sTils irrespective of lymphocyte subset is associated with a better prognosis. In addition, the standardized assessment of sTils provides a quantitative and a more tangible picture of the extent of immune activation in BRCA breast cancers.
Similar to sporadic breast cancers, sTils levels varied according to ER status, with the largest proportion of sTils in ER negative breast cancers. Importantly, this was observed in both BRCA1 and BRCA2 breast cancers. Stromal Tils have only been associated with a better prognosis in TN and HER2 positive subgroups among sporadic breast cancers. The majority of BRCA1 breast cancers were ER negative (67%), and the majority of BRCA2 breast cancers were ER positive (82%). This may indicate that the large proportion of ER negative breast cancers among BRCA1 carriers mostly drove the observed significant association of sTils with prognosis in the BRCA1 subgroup. A recent study reported worse survival with each 10% increase in sTils levels in luminal-like subtype breast cancers. However they also showed that the prognostic impact of sTils in luminal-like breast cancers varied according to clinico-pathological factors, and a positive prognosis was observed in histological high-grade luminal-like tumors [Citation13]. To assess if sTils are prognostic in subgroups of ER positive and ER negative breast cancers within BRCA1 and BRCA2 breast cancer subgroups, test of interactions are required. However, there is not enough power to detect an interaction by subgroup on the level of both affected BRCA gene and ER status. It might be fair to speculate that sTils are associated with improved survival in a subgroup of ER positive breast cancers since BRCA-mutated breast cancers are associated with high histological grade.
Pathological factors are incorporated in risk prediction models (e.g., BOADICEA [Citation32]) and differences in sTils levels according to BRCA gene and intrinsic subtype may prove useful to predict BRCA mutation status among breast cancer patients with unknown carrier status. For instance, high sTils were observed in a rather high amount of ER positive BRCA2-mutated breast cancers, and high sTils in ER positive breast cancer may predict BRCA2 status, however the utility of this is only speculative based on the observed high sTils in this cohort. Evaluation of differences of sTils among BRCA2 breast cancers compared to a control group of patients with no BRCA mutation and additionally investigation of the predictive value of sTils incorporated into risk assessment models are required to establish such utility.
The strengths of this study are the large sample size of BRCA-mutated breast cancers, and the standardized method of sTils assessment. The nationwide BRCA population and collection of archived FFPE breast cancer tissue makes this the largest study of evaluation of sTils in BRCA-mutated breast cancers to date.
Although the IOBWG recommendations for Tils evaluation are the most well-investigated assessment method for Tils, the method still needs analytical validation mainly because of inter-observer variability [Citation33]. This may have affected the assessed distribution of sTils groups. Other potential limitations are the selection for BRCA testing, causing results to be inferred only to BRCA carriers selected by the similar criteria. In addition, variable time-span between breast cancer surgery and BRCA-test introduced survivorship bias however this was minimized by including only follow-up time beyond BRCA test date.
Previously, Tils in BRCA breast cancer have been described semi-quantitatively as ‘sparse’ or ‘prominent’ [Citation1]. However, using the IOBWG guidelines, one recent study has compared sTils in twenty-nine BRCA1-mutated and sixty-four sporadic TN breast cancers, and reported a significantly higher amount of sTils in BRCA1-mutated breast cancers [Citation34]. In addition, they also found a significantly higher mutation frequency in BRCA1 versus sporadic breast cancers supporting the hypothesis of a higher immunogenicity of BRCA breast cancers because of higher mutational burden - at least for BRCA1-mutated TN/basal-like subtype.
In conclusion, BRCA breast cancers shows high activation of the immune system and increasing amount of sTils are associated with lower mortality and better DFS in BRCA carriers. Importantly, a high proportion of BRCA breast cancers have high sTils underpinning the importance of Tils assessment in BRCA-mutated breast cancers. Stromal Tils should be considered adjusted for in future survival studies comparing BRCA-mutated and sporadic breast cancers. Investigation of the composition of the lymphocyte subsets and activated immune regulatory pathways, and in addition, any benefit from immune-modulatory treatment in association with sTils and BRCA-mutation, would be interesting to explore in future studies.
Supplemental Material
Download MS Word (18.1 KB)Disclosure statement
AMG participated in an advisory board meeting about BRCA testing in ovarian cancers in February 2016, funded by AstraZeneca.
Additional information
Funding
References
- Lakhani SR, Jacquemier J, Sloane JP, et al. Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst. 1998;90:1138–1145.
- Waddell N, Arnold J, Cocciardi S, et al. Subtypes of familial breast tumours revealed by expression and copy number profiling. Breast Cancer Res Treat. 2010;123:661–677.
- Massink MP, Kooi IE, van Mil SE, et al. Proper genomic profiling of (BRCA1-mutated) basal-like breast carcinomas requires prior removal of tumor infiltrating lymphocytes. Mol Oncol. 2015;9:877–888.
- Mavaddat N, Barrowdale D, Andrulis IL, et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomark Prevent. 2012;21:134–147.
- Armes JE, Egan AJ, Southey MC, et al. The histologic phenotypes of breast carcinoma occurring before age 40 years in women with and without BRCA1 or BRCA2 germline mutations: a population-based study. Cancer. 1998;83:2335–2345.
- Marcus JN, Watson P, Page DL, et al. Hereditary breast cancer: pathobiology, prognosis, and BRCA1 and BRCA2 gene linkage. Cancer. 1996;77:697–709.
- Marcus JN, Watson P, Page DL, et al. BRCA2 hereditary breast cancer pathophenotype. Breast Cancer Res Treat. 1997;44:275–277.
- Agnarsson BA, Jonasson JG, Bjornsdottir IB, et al. Inherited BRCA2 mutation associated with high grade breast cancer. Breast Cancer Res Treat. 1998;47:121–127.
- Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. JCO. 2015;33:983–991.
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (London, England). 2014;384:164–172.
- Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. JCO. 2013;1;31:860–867.
- Dieci MV, Mathieu MC, Guarneri V, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol. 2015;26:1698–1704.
- Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50.
- Mittal D, Gubin MM, Schreiber RD, et al. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25.
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science (New York, NY). 2011;331:1565–1570.
- Efremova M, Finotello F, Rieder D, et al. Neoantigens generated by individual mutations and their role in cancer immunity and immunotherapy. Front Immunol. 2017;8:1679.
- Strickland KC, Howitt BE, Shukla SA, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7:13587–13598.
- Stovgaard ES, Nielsen D, Hogdall E, et al. Triple negative breast cancer – prognostic role of immune-related factors: a systematic review. Acta Oncol. 2018;57:74–82.
- Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an international TILs working group 2014. Ann Oncol. 2015;26:259–271.
- Christiansen P, Ejlertsen B, Jensen MB, et al. Danish breast cancer cooperative group. Clin Epidemiol. 2016;8:445–449.
- Soenderstrup IMH, Laenkholm AV, Jensen MB, et al. Clinical and molecular characterization of BRCA-associated breast cancer: results from the DBCG. Acta Oncol. 2018;57:95–101.
- DBCG [Internewt]. Clinical guidelines. 2017. Available from: http://dbcg.dk/, accessed March 20, 2018.
- Schmidt M, Schmidt SA, Sandegaard JL, et al. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490.
- Bjerregaard B, Larsen OB. The Danish pathology register. Scand J Public Health. 2011;39:72–74.
- Gjerstorff ML. The Danish cancer registry. Scand J Public Health. 2011;39:42–45.
- Pedersen IS, Schmidt AY, Bertelsen B, et al. A Danish national effort of BRCA1/2 variant classification. Acta Oncol. 2017;23:1–3.
- Thomassen M, Hansen TV, Borg A, et al. BRCA1 and BRCA2 mutations in Danish families with hereditary breast and/or ovarian cancer. Acta Oncol. 2008;47:772–777.
- Lakhani SREI, Schnitt SO, et al. WHO classification of tumours of the breast. 4th ed. Vol. 4. France: WHO/IARC; 2012.
- Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11:359–377.
- Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. JCO. 2010;28:2784–2795.
- Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. JCO. 2013;31:3997–4013.
- Lee AJ, Cunningham AP, Kuchenbaecker KB, et al. BOADICEA breast cancer risk prediction model: updates to cancer incidences, tumour pathology and web interface. Br J Cancer. 2014;110:535–545.
- Tramm T, Di Caterino T, Jylling AB, et al. Standardized assessment of tumor-infiltrating lymphocytes in breast cancer: an evaluation of inter-observer agreement between pathologists. Acta Oncol. 2018;57:90–94.
- Nolan E, Savas P, Policheni AN, et al. Combined immune checkpoint blockade as a therapeutic strategy for BRCA1-mutated breast cancer. Sci Trans Med 2017;9:pii: eaal4922.