Abstract
Background: Blood-based, cancer-associated biomarkers may detect subjects at risk of having neoplastic diseases. The aim of the present study was to evaluate whether elevated serological protein biomarker levels may identify adenoma patients, who are at increased risk of being diagnosed with subsequent primary malignancy.
Methods: Levels of CEA, CA19-9, TIMP-1 and YKL-40 were determined in blood samples collected prior to diagnostic bowel endoscopy due to symptoms of colorectal neoplasia. Follow-up time was ten years, and identified adenoma patients, who were diagnosed with subsequent primary intra- or extra-colonic malignant diseases. The biomarker levels were also determined in 400 subjects, who underwent diagnostic colonoscopy, had clean colorectum and were without apparent co-morbidity; these levels were used as reference levels. In the present study, biomarkers were interpreted as elevated when levels were above the reference intervals adjusting for age and gender. The 1-year and 5-years cumulative incidences were calculated.
Results: Primary malignancies were identified in 175 (19%) of the 923 subjects diagnosed with adenomas at the primary bowel endoscopy. In detail, 20 of the 175 subjects were diagnosed with colorectal cancer (CRC) and 155 subjects with extra-colonic cancers. Thirty patients were diagnosed with malignancy within the first year. Three groups were established: 0: no elevated biomarkers; 1: 1 of the 4 biomarkers elevated; and 2: ≥2 biomarkers elevated. The cumulative 5-years incidence of malignancy was: 0: 6.9%; 1: 11.8%; and 2: 17.5% (p = .0009).
Conclusion: Elevated blood-based, cancer-associated protein biomarker levels in subjects diagnosed with adenomas at large bowel endoscopy identifies subjects at increased risk of being diagnosed with subsequent primary malignancy.
Introduction
Colorectal cancer (CRC) is diagnosed in more than 1.36 million patients and more than 694.000 die from the disease worldwide every year [Citation1]. Excluding skin cancers, CRC is the second most common cancer in women and the third most common cancer in men [Citation1]. The incidence varies from country to country, but certainly is age-dependent [Citation2]; the median age when diagnosed with CRC is approximately 70 years [Citation2,Citation3]. Most CRCs arise via the adenoma-carcinoma sequence [Citation4], and therefore early detection and removal of potential premalignant lesions is of utmost importance [Citation5]. Furthermore, the detection of CRC in early stages most often leads to complete cure with long-term survival [Citation5].
Colorectal polyps and adenomas are often found at large bowel endoscopy performed due to symptoms attributable to CRC, or as part of a screening program. In a recent European multinational study, polyps were found in 48% of the examined subjects at colonoscopy, and adenomas were found in 31% of the subjects, of which 10% were high risk adenomas [Citation6]. The prevalence of polyps varies between 30% and 58% in different studies, and the reported prevalence of adenomas is closely related to the prevalence of polyps [Citation6–9]. Most subjects have asymptomatic adenomas, but some adenomas are associated with symptoms such as bleeding, changed bowel movement habits, abdominal pain and incomplete bowel evacuation. The symptoms vary due to size, type and location of the adenomas. Some adenomas, depending on morphology and histology, are premalignant and those adenomas will potentially transform to CRC if undetected. When a subject has had low risk adenomas previously removed, which is common in adults over 50 years of age, the risk of developing advanced adenomas is significantly increased [Citation10]. Less than ten percent of the adenomas progress to CRC [Citation11], but development of CRC appears as a slow process of 10 to 15 years [Citation12,Citation13], which may lead to sufficient time to identify such subjects by screening and have the lesions excised. About 80% of CRCs are sporadic and the remaining 20% have a genetic component such as detected in familial adenomatous polyposis and hereditary non-polyposis colorectal cancer (HNPCC) [Citation14,Citation15].
Various methods to detect CRC and premalignant lesions have been introduced over the last decades. Sigmoidoscopy has previously been used as an examination of subjects with symptoms of a left sided colorectal neoplasm, but sigmoidoscopy does not reach the transverse and the right side of the colon, and thereby many neoplastic lesions will be missed [Citation16,Citation17]. At present, a subject with symptoms attributable to CRC will be offered colonoscopy to visualize the entire bowel. In current screening for CRC, the common standard in most European countries is Fecal Immunochemical Testing (FIT) followed by colonoscopy of subjects with a FIT positive result [Citation18]. Although diagnostic and screening colonoscopies lead to identification of subjects with adenomas that subsequently are offered frequent adenoma-control colonoscopies due to the risk of new neoplastic bowel lesions including cancers [Citation19], it should be considered that those subjects still may be at risk of developing extra-colonic cancers without any association with the adenomas. The adenoma-control procedure does not include examinations for extra-colonic lesions, and thereby such lesions may be missed although some subjects report symptoms at the adenoma control colonoscopy.
Serological cancer-associated protein biomarker levels have uniformly been demonstrated elevated in subjects identified with CRC [Citation3]. Recent results indicate that such protein biomarker levels may, however, also be elevated in some subjects with clean colorectum [Citation3,Citation20] and in subjects with benign findings, such as diverticula, at large bowel endoscopy [Citation20,Citation21]. In addition, results indicate that elevated levels of some cancer-associated, protein biomarkers identify otherwise healthy subjects at risk of developing a primary malignant disease [Citation20]. The biomarker levels may be elevated due to physiological changes, but possibly also due to processes that are leading to development of various diseases including malignancy as presented previously [Citation20,Citation21]. The aim of the present study was to evaluate a potential association between elevated levels of cancer-associated serological protein biomarkers CEA, CA19-9, TIMP-1 and YKL-40 in subjects diagnosed with adenomas at large bowel endoscopy and subsequent diagnosis of primary malignancy.
Material and methods
In a major multicenter study conducted in 2004/05 at six collaborating hospitals in Denmark, 4990 subjects were included in a prospective, population-based cohort study and underwent diagnostic large bowel endoscopy due to symptoms attributable to CRC. Blood samples were collected prior to endoscopy and plasma levels of the protein biomarkers CEA, CA19-9, TIMP-1 and serum YKL-40 were retrospectively determined to identify a potential relation to endoscopy findings. The four biomarkers were chosen because they showed promising results as neoplasia detecting biomarkers in previous studies [Citation3,Citation22]. The subjects were stratified into five ranked groups depending on the primary endoscopy findings: 1: CRC; 2: extra-colonic cancer; 3: colorectal adenomas; 4: non-neoplastic findings, mainly diverticula and 5: clean colorectum. The study was approved by the Ethics Committee of the Capital Region of Denmark (KF01-080/03), which represents all collaborating hospitals, and in addition approved by the Danish Data Protection Agency (2003-41-3312/2008-41-2252). The inclusion and exclusion criteria of the subjects have been presented previously [Citation3]. Before inclusion, all subjects signed an informed consent form. Symptoms or adherence to a HNPCC surveillance program guided whether a subject underwent sigmoidoscopy or colonoscopy. Subjects with findings of adenomas at primary sigmoidoscopy should have been offered subsequent colonoscopy; that was not uniformly performed however, due to patient refusal. Subjects, who underwent primary sigmoidoscopy without findings of colorectal pathology, but with persisting symptoms were offered an additional colonoscopy. Subjects, who had been examined with primary colonoscopy or examined with primary sigmoidoscopy and subsequent colonoscopy but had persisting symptoms were offered additional examinations with ultra-sonography, computerized axial tomography or magnetic resonance imaging.
Blood samples for EDTA plasma and serum were collected from all subjects just prior to endoscopy and were handled and subsequently frozen at −80 °C under electronic 24/7 surveillance, according to a validated standard operative procedure [Citation3], which was finalized within two hours of blood collection. The inclusion of subjects ended 31 December 2005. Audits were performed both on-site and electronically via patient files and existing databases. After termination of the study inclusion, the levels of the 4 protein biomarkers were analyzed at commercially available and well validated analysis platforms. CEA, CA19-9 and TIMP-1 were determined in EDTA plasma using the Abbott ARCHITECT® i2000 automated immunoassay system [Citation3]. The levels of the cancer-associated biomarker YKL-40 were determined in serum by a commercial ELISA platform [Citation21] with an on-site detection limit 10 ng/mL, intra-assay coefficients of variability (CV) of 5% and inter-assay CV of <6%.
All findings at endoscopy were recorded in an approved database using the International Coding for Diseases-10 (ICD-10) system. In addition, all subjects had gender, age, BMI and current comorbidity recorded. The adenomas found at endoscopy were stratified by the current classification as either at high- or low-risk. If one or more of the following parameters were met, the adenomas were classified as at high-risk: ≥1 cm in diameter, ≥3 lesions, villous histology or high-grade dysplasia. Low-risk adenomas were classified as follows: <1 cm in diameter, <3 lesions, tubular histology or low-grade dysplasia. The analysis results of the individual and the combined biomarkers were compared to database information about subsequent diagnosis of primary malignant disease in the defined adenoma subgroup. Skin cancers except malignant melanoma were not included. Subjects diagnosed with adenomas at endoscopy were followed prospectively from primary endoscopy in 2004/05 to the cut point 31.12.2014 corresponding to 9 to 10 years follow up period.
Statistics
Descriptive statistics were presented by the median, minimum, maximum and quartiles for continuous data. The Spearman rank correlation was used as a measure of association, and tests for comparing biomarker levels between strata were done using the Wilcoxon rank sum test. Comparisons between strata with adjustment for age and gender were performed using a linear model with the biomarker levels log transformed. The latter results were presented by the relative differences (ratio) with 95% confidence intervals. The elapsed time to diagnosis of a malignant disease and the association to the biomarker levels at the endoscopy were analyzed with death as a competing risk [Citation23]. Each biomarker level has been defined as normal or elevated based on the 90th percentile of age and gender adjusted reference intervals. The reference intervals for each biomarker were constructed by regressing the logarithm of each biomarker on age and gender using a cohort of 400 subjects that underwent colonoscopy, but subsequently was shown to be without any colorectal findings, without known co-morbidity and without known medication intake. The choice of the 90th percentile as threshold was done prior to analysis in order to have sufficient power for discrimination, choosing the 95th percentile instead showed a similar result (data not shown). Three groups were established: 0: no elevated biomarkers; 1: 1 of the 4 biomarkers elevated; and 2: ≥2 biomarkers elevated. Univariable analyses of time to diagnosis of primary cancer have been done for each biomarker in addition to multivariable analysis showing the association for subjects with no elevated (group 0), one elevated (group 1) or two or more elevated (group 2) biomarkers. All subsequent primary cancers recorded in the observation period were included (except non-melanoma skin cancers), and the cumulative incidence rate was calculated after 1 and 5 years, respectively. The one-year cumulative incidence was chosen to evaluate the number of subjects, who possibly may have had a malignant disease at the time of primary endoscopy, but which would be missed because the adenoma diagnosis that would be sufficient to explain the symptoms. The 5-year cumulative incidence was chosen to evaluate the number of subjects had developed a malignant disease after the primary endoscopy. p values <5% were considered statistically significant. Finally, the NORDCAN database has been used for calculation of the overall age and gender matched cancer incidence (except skin cancers) of the Danish population in 2004–2005.
Results
Of the 4990 included subjects, 923 were diagnosed with primary adenomas at large bowel endoscopy. Of these, 409 (44.3%) were women with a median age of 64 (20–91) years and 514 were men with the median age of 63 (20–92) years. Two of the 923 subjects had insufficient data recorded at inclusion and were excluded from the calculations. The adenoma subgroup included 62 subjects with HNPCC. During the observation period from 2004/05 until the cut point 31.12.2014, 175 (19.0%) of the subjects diagnosed with adenomas were subsequently diagnosed with a primary malignant disease. The median age of the subjects, who were diagnosed with a primary malignant disease was 67 (25–88) years at the time of the diagnosis.
The cumulative probability of being diagnosed with any malignancy after primary large bowel endoscopy at 1, 2, 3, 4 and 5 years was 3.3% (CI: 2.1–4.4), 4.9% (CI: 3.5–6.3), 7.8% (CI: 6.0–9.4), 8.8% (CI: 6.9–10.6) and 10.6% (CI: 8.6–12.6), respectively. A primary malignant disease was diagnosed in 30 subjects within 12 months after endoscopy, in 68 subjects between 12 and 60 months after endoscopy and in 77 subjects from 60 months after endoscopy until the cut point 31.12.2014 (). Of the 30 subjects, who were diagnosed with primary malignancy within the first year, 10 subjects did not have any of the four biomarkers elevated, 7 subjects had one elevated biomarker, 8 subjects had two biomarkers elevated, 3 subjects had 3 biomarkers elevated and 2 subjects had all four biomarkers elevated. In total, 20 subjects developed CRC in the follow up period and 4 of these subjects were diagnosed with cancer within the first 12 months. One of the 4 subjects underwent sigmoidoscopy as primary endoscopy; the subsequent registered lesion was unspecified colonic cancer. Fourteen of the 20 subjects, who developed CRC, had low-risk adenomas, and 6 subjects had high-risk adenomas at primary endoscopy. Eight of the 20 CRC subjects were diagnosed CRC in the same segment of the colorectum as they previously had had an adenoma lesion removed. shows the different anatomic locations of CRC and diagnostic approach at inclusion.
Table 1. Cancer location and time to primary malignancy.
Table 2. Primary colorectal examination.
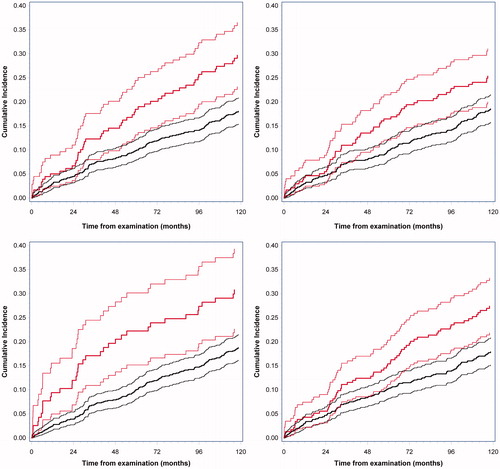
Comparison of the specific biomarker levels between subjects with at high- and low-risk adenomas did not show significant differences: CEA: p = .27; CA19-9: p = .20; TIMP-1; p = .17; and YKL-40: p = .18. shows the number of subjects, who had elevated levels of any of the four biomarkers just prior to the primary endoscopy, and the 1 and 5 years cumulative incidences.
Table 3. Subjects with elevated cancer-associated biomarkers.
Univariable analyses showed that levels of all four cancer-associated biomarkers CEA (p = .0008), CA19-9 (p = .002), TIMP-1 (p = .02) and YKL-40 (p = .002) were significantly associated with development of malignancy. If the 62 HNPCC patients were excluded from the adenoma population, the p-values were almost similar: CEA (p = .003), CA19-9 (p = .004), TIMP-1 (p = .023) and YKL-40 (p = .008). A sensitivity analysis for incidence of all 4 biomarkers applied to those individuals where all events occurred within the first year were removed, demonstrated that CEA, TIMP-1 and YKL-40 remained significant (p = .012, p = .047 and p = .007, respectively) whereas CA19-9 was not significant (p = .28).
The cumulative 1-year and 5-year incidences for the four biomarkers are listed in and are shown in , respectively. A multivariable analysis including all biomarkers showed that increased levels of one or more biomarkers were significantly associated with development of subsequent malignancy (p = .0009). Multivariable analysis demonstrated that 3 of the 4 included biomarkers were statistically significant (CEA, CA19-9 and YKL-40) in combination whereas TIMP-1 was not (p = .64).
Figure 1. Cumulative incidence of malignancy according to plasma/serum levels of A: CEA (p = .0008), B: TIMP-1 (p = .02), C: CA19-9 (p = .002) and D: YKL-40 (p = .002). The solid blue lines represent normal levels of the cancer-associated biomarkers with 95% CI. The dotted red lines represent elevated levels of the cancer-associated biomarkers with 95% CI.
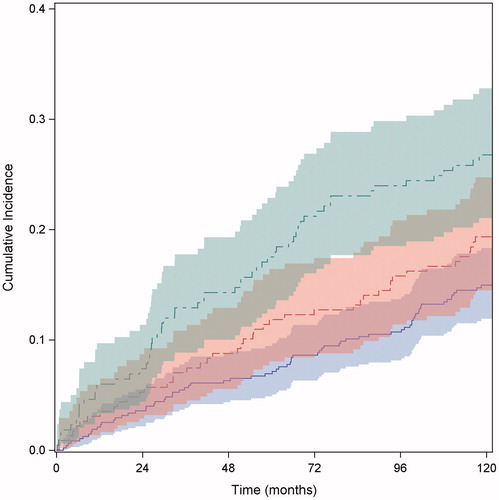
The 1-year cumulated risk of being diagnosed with a primary malignant disease in the three predefined groups was: group 0: 2.1% (1.1–3.7); group 1: 3.1% (1.4–5.9); and group 2: 6.0% (3.4–9.7), respectively (). The 5-years cumulated risk of being diagnosed with a primary malignant disease in the three predefined groups were; group 0: 6.9% (4.9–9.5); group 1: 11.8% (8.0–16.4); and group 2: 17.5% (12.8–22.9), respectively (). For comparison, the overall cumulated cancer incidence of the age and gender matched entire Danish population was 2% at one year and 8% at five years, respectively.
Figure 2. Cumulative incidence of malignancy according to the multivariate analysis including the three defined groups (p = .0009). The solid blue line with 95% CI, represents group 0 with none of the four biomarkers elevated. The dotted red line with 95% CI, represents group 1, with one of the four biomarkers elevated. The dotted green line with 95% CI, represents group 2, with two or more of the four biomarkers elevated.
At inclusion, information on co-morbidity was recorded in all 4990 subjects, but since it was shown previously that co-morbidities do not influence the risk of subsequent malignant diagnosis [Citation3,Citation20], the co-morbidities have not been considered in the present study.
Discussion
The overall results of the present study show that 19% of subjects, who were diagnosed with colorectal adenomas at diagnostic large bowel endoscopy, subsequently and within a 9–10 years period were diagnosed with a primary malignant disease. The findings are in accordance with previous results among subjects with clean colorectum [Citation20] or with diverticula at diagnostic endoscopy [Citation21]. The present results also confirm that the risk of being diagnosed with subsequent malignancy disease was time-dependent. The majority (155) of the 175 subjects were diagnosed with extra-colonic cancers, although they were initially included in the study due to symptoms of CRC. Within the first year four subjects were subsequently diagnosed with CRC and 26 subjects with extra-colonic cancers, although they were diagnosed with adenomas at the primary endoscopy. The diagnoses were based on additional examinations due to return to the out-patient clinics with persisting symptoms.
Although it is well accepted that subjects, who have been diagnosed with adenomas are at risk of developing CRC [Citation19] and therefore are under surveillance, only 20 of the 175 with a subsequent malignant diagnosis developed CRC; four of those were diagnosed within the first year of follow-up. One of the four subjects had only been examined with sigmoidoscopy at inclusion and the location of the colon cancer was recorded as unspecified due to inability to undergo resection. The three other subjects were undergoing colonoscopy at inclusion, and were subsequently diagnosed with cancer in the ascending, the transverse and the sigmoid colon, respectively (). The findings indicate that those cancer lesions may have been missed at the primary colonoscopy. Among the remaining 16 subjects, who developed CRC in the follow-up period, 9 subjects developed a right sided colonic cancer. Three of those 9 subjects only underwent sigmoidoscopy at the inclusion. Thus, precancerous lesions or manifest cancers may have been missed at inclusion due to only partial examination of the colon. In addition, the 26 subjects, who were diagnosed with extra-colonic cancers within the first year, may have had the disease at the primary endoscopy. shows that most of those cancers may be categorized as differential diagnoses to CRC and the symptoms thereby may have led to the endoscopy; indeed a few cancer diseases, such as tonsil and brain cancers may have had non-characterized symptoms that led to endoscopy. The results of the 26 subjects may lead to future considerations on subjects referred to diagnostic colonoscopy, specifically with subsequent findings of adenomas, which may explain the symptoms. That the diagnosis in few instances may blur the real disease that prompted the bowel examination. At present, the available results of biomarker examinations added to colonoscopy is not sufficient, however, to recommend a blood test in addition to diagnostic colonoscopy. Future results from major studies may lead to such considerations [Citation24].
Blood samples were not collected after adenoma/polyp removal to verify possible reduction in the protein biomarker levels. Subsequent studies need to include such analyses to evaluate whether the increased levels are due to presence of the adenomas or might be genuinely increased levels due to an ongoing malignancy development process.
Univariate analyses of the four serological, cancer-associated protein biomarkers, determined in the entire group of subjects, who subsequently was diagnosed with a malignant disease, were all significantly and time-dependently associated with risk of development of primary malignancy. These findings are consistent with results of two previous studies [Citation20,Citation21], and appear to validate the findings that increased protein biomarker levels may identify subjects at risk of being diagnosed with malignancy [Citation25–27].
A subpopulation of 62 subjects was included in the study, although they were adhering to the current Danish HNPCC surveillance program. Those 62 subjects, often younger than the median age at inclusion of 64 years are, as very well accepted, at higher risk of developing CRC. Additional univariable analyses of the 4 cancer-associated biomarkers were performed with the exclusion of the HNPCC surveillance subjects due to their higher risk of colorectal malignancy. Those univariable analyses showed that all 4 biomarkers significantly identified subjects at risk of malignancy, and when the p values were compared to the previously calculated p values there were only minor differences. Because of the comparable univariable analyses, the influence of the HNPCC subjects has not been considered in the remaining analyses.
The multivariable analysis indicated that increased levels of one or more of the 4 biomarkers were significantly associated with increased risk of being diagnosed with subsequent primary malignancy, including CRC, which also corresponds to results of two previous studies [Citation20,Citation21].
Although the results of the present study appear to underline the results of the two previous studies [Citation20,Citation21], it should be considered however, that the three different, specific study populations were selected from the same overall study population [Citation3]. Thus, these results should be finally validated in independent study populations that are sufficiently powered to confirm or reject the present findings. In addition, combinations of cancer-specific protein biomarkers and inclusion of additional genetic, epigenetic and/or metabolomics biomarkers to the combination may add to the efficacy of identifying subjects at increased risk of being diagnosed with subsequent malignancy, including CRC, after colonoscopy without findings of CRC [Citation28–30]. Such future combinations may lead to specific risk stratifications through algorithmic calculations based on the number and specificity of elevated biomarkers. Thus, a subject with elevated cancer-associated biomarkers may get different specific cancer risks calculated, and that may be of benefit to target the future cancer search [Citation31,Citation32].
Conclusion
Subjects with findings of adenomas at diagnostic large bowel endoscopy and elevated circulating levels of the protein biomarkers CEA, CA-19-9, TIMP-1 and YKL-40 appear to be at increased risk of being subsequently diagnosed with various malignant diseases, including CRC. Significant differences between the biomarker levels in subjects with at high- or low-risk adenomas could not be shown, which indicate that the increased biomarker levels may be associated with the presence or the risk of subsequent malignancy. In addition, the risk of malignancy appears to be time-dependently increased and associated with the number of elevated serological cancer-associated protein biomarkers.
Acknowledgments
The research nurses, secretaries and technicians at the participating hospital departments and laboratories are thanked for their skillful work.
Disclosure statement
Determinations of CEA, CA19-9 and TIMP-1 in plasma samples were performed at Abbott Centre’s of Excellence in Munich, Germany and Amsterdam the Netherlands free of charge. Gerard Davis is an employee of Abbott Laboratories Inc., Abbott Park, IL, USA.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
- Brenner H, Kloor M, Pox CP. Colorectal Cancer. Lancet. 2014;383:1490–1502.
- Nielsen HJ, Brünner N, Jørgensen LN, et al. Plasma TIMP-1 and CEA in detection of primary colorectal cancer: a prospective, population based study of 4509 high-risk individuals. Scand J Gastroenterol. 2011;46:60–69.
- Singh R, Zorrón Cheng LTP, Koay D, Burt A. Sessile serrated adenoma/polyps: where are we at in 2016?. World J Gastroenterol. 2016;22:7754–7759.
- Van der Stok EP, Spaander MCW, Grünhagen DJ, et al. Surveillance after curative treatment for colorectal cancer. Nat Rev Clin Oncol. 2017;14:297–315.
- Bretthauer M, Kaminski MF, Løberg M, et al. Population-based colonoscopy screening for colorectal cancer: a randomized clinical trial. JAMA Intern Med. 2016;176:894–899.
- Waldmann E, Heinze G, Ferlitsch A, et al. Risk factors cannot explain the higher prevalence rates of precancerous colorectal lesions in men. Br J Cancer. 2016;115:1421–1429.
- Rex DK, Helbig CC. igh Yields of small and flat adenomas with high-definition colonoscopes using either white light or narrow band imaging. Gastroenterology. 2007;133:42–47.
- IJspeert JEG, van Doorn SC, van der Brug YM, et al. The proximal serrated polyp detection rate is an easy-to-measure proxy for the detection rate of clinically relevant serrated polyps. Gastrointest Endosc. 2015;82:870–877.
- Dube C, Yakubu M, McCurdy BR, et al. Risk of advanced adenoma, colorectal cancer, and colorectal cancer mortality in people with low-risk adenomas at baseline colonoscopy: A systematic review and meta-analysis. Am J Gastroenterol. 2017;112:1790–1801.
- Levine JS, Ahnen DJ. Clinical practice. Adenomatous polyps of the colon. N Engl J Med. 2006;355:2551–2557.
- Kelloff GJ, Schilsky RL, Alberts DS, et al. Colorectal adenomas: a prototype for the use of surrogate endpoints in the development of cancer prevention drugs. Clin Cancer Res. 2004;10:3908–3918.
- Bretthauer M, Kalager M, Adami O. Do's and don'ts in evaluation of endoscopic screening for gastrointestinal cancers. Endoscopy. 2016;48:75–80.
- Trimbath JDT, Giardiello FM. Review article: genetic testing and counselling for hereditary colorectal cancer. Aliment Pharmacol Ther. 2002;16:1843–1857.
- Kennelly RP, Gryfe R, Winter DC. Familial colorectal cancer: patient assessment, surveillance and surgical management. Eur J Surg Oncol. 2017;43:294–302.
- Lieberman DA, Harford WV, Ahnen DJ, et al. Veterans Affairs Cooperative Study Group 380. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med. 2001;345:555–560.
- Niedermaier T, Weigl K, Hoffmeister M, et al. Flexible sigmoidoscopy in colorectal cancer screening: implications of different colonoscopy strategies. Eur J Epidemiol. 2018;33:473–484.
- Nielsen HJ, Christensen IJ, Andersen B, et al. Serological biomarkers in triage of FIT-positive subjects?. Scand J Gastroenterol. 2017;52:742–744.
- Løberg M, Kalager M, Holme Ø, et al. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med. 2014;371:799–807.
- Kring TS, Piper TB, Jørgensen LN, et al. Blood-based biomarkers at large bowel endoscopy and prediction of future malignancies. Biomark Cancer. 2015;7:57–61.
- Hvolris MH, Piper TB, Hammer E, et al. Increased serological cancer-associated biomarker levels at large bowel endoscopy and risk of subsequent primary cancer. Scand J Gastroenterol. 2016;51:860–865.
- Johansen JS, Christensen IJ, Jørgensen LN, et al. Serum YKL-40 in risk assessment for colorectal cancer: a prospective study of 4,496 subjects at risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2015;24:621–626.
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
- Rasmussen L, Wilhelmsen M, Christensen IJ, et al. Protocol outlines for part 1 and 2 of the prospective Endoscopy III study for the early detection of colorectal cancer: validation of a concept based on blood biomarkers. JMIR Res Protoc. 2016;5:e182.
- Allin KH, Bojesen SE, Johansen JS, et al. Cancer risk by combined levels of YKL-40 and C-reactive protein in the general population. Br J Cancer. 2012;106:199–205.
- Kjaergaard AD, Nordestgaard BG, Johansen JS, et al. Observational and genetic plasma YKL-40 and cancer in 96,099 individuals from the general population. Int J Cancer. 2015;137:2696–2704.
- Allin KH, Bojesen SE, Nordestgaard BG. Inflammatory biomarkers and risk of cancer in 84,000 individuals from the general population. Int J Cancer. 2016;139:1493–1500.
- Holdenrieder S, Dharuman Y, Standop J, et al. Novel serum nucleosomic biomarkers for the detection of colorectal cancer. Anticancer Res. 2014;34:2357–2362.
- Phallen J, Sausen M, Adleff V, et al. Direct detection of early stage cancers using circulating tumor DNA. Sci Transl Med. 2017;9:eaan2415.
- Schøler LV, Reinert T, Ørntoft MW, et al. Clinical implications of monitoring circulating tumor DNA in patients with colorectal cancer. Clin Cancer Res. 2017;23:5437–5445.
- Cohen JD, Li L, Wang Y, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test . Science. 2018;359:926–930.
- Wilhelmsen M, Christensen IJ, Jørgensen LN, et al. Clean colorectum at diagnostic colonoscopy: subsequent detection of extra-colonic malignancies by plasma protein biomarkers? Biomark Cancer. 2018;10:1179299X1877697.
