Abstract
Objective: Inflammation has been validated as a host-related prognostic marker in cancer. The Glasgow Prognostic score (GPS) and neutrophil-to-lymphocyte ratio (NLR) are suggested measures of inflammation. However, the allocation of patients has been questioned. Hence, optimized inflammation-scores has been developed, such as the combined NLR and GPS (CNG) system, and the Aarhus composite biomarker score (ACBS). So far, these optimized inflammation-scores have not been validated in lung cancer patients. We evaluated if the optimized inflammation-scores were prognostic markers of inferior survival in lung cancer patients. Furthermore, we tested which of the optimized inflammation-scores led to better patient-allocation.
Material and methods: The cohort of this prospective study composed of 275 non-small cell lung cancer patients. We evaluated pre-diagnostic serum biomarkers for GPR, NLR, platelet-to-lymphocyte ratio as well as the optimized inflammation-scores CNG and ABCS as predictors of overall survival (OS), and we examined the patient-allocation derived from each inflammation-score.
Results: Each of the evaluated inflammation-scores could predict the overall survival even when adjustments were made for comorbidity and clinicopathological characteristics. When comparing the scores, the optimized inflammation-scores CNG and ACBS led to a better and more balanced patient-allocation. In the early clinical stages I & II, the optimized scores could reveal a subgroup of patients with poorer survival that is similar to stage III.
Conclusion: In this cohort of lung cancer patients, we demonstrate that inflammation-scores are prognostic markers of inferior survival. Furthermore, we demonstrate that the optimized inflammation-scores CNG and ACBS lead to better patient-allocation independently of the clinicopathological characteristics and comorbidity.
Background
Over the last decades, many prognostic markers have been suggested in lung cancer, but only very few have proven to be clinically relevant. This is disappointing since lung cancer remains a leading cause of cancer-related death [Citation1,Citation2]. In order to improve lung cancer survival, we need prognostic markers that can identify patients who are at high risk of inferior survival. By identifying these patients, we can facilitate stratification into optimal treatment regimens or follow-up programs and hopefully improve survival for the lung cancer patients. In general, prognostic markers can be classified as either tumor- or host-related. The most widely accepted tumor-related prognostic marker is the tumor, node and metastasis (TNM) staging system [Citation3]. Based on the TNM staging system, patients are classified in clinical or pathological stages and stratified for treatment regimens. But the prognosis within each stage can vary tremendously. So far, the only recognized host-related prognostic marker is the WHO performance status (PS), which categorizes patients based on daily life activities [Citation4].
Inflammation is now recognized as one of the hallmarks of cancer [Citation5] and therefore inflammation has been suggested as another host-related prognostic marker [Citation6]. General inflammation markers like C-reactive protein (CRP), leucocytes and lymphocytes have shown some perspectives as prognostic markers of inferior survival in cancer [Citation7,Citation8]. However, using the markers individually as a measure of the inflammation status is a simplistic approach to a complex problem. Hence, inflammation scores that combines more than one parameter such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and Glasgow Prognostic Score (GPS), which is based on albumin and CRP, have been suggested as a more representative marker of the inflammatory process. Indeed, when these scores were tested they proved to be prognostic in several cancers [Citation8–15]. In lung cancer, the GPS is prognostic of inferior survival independently of stage and histology but it is only able to identify a smaller proportion of the patients with a decreased survival [Citation11,Citation16–18]. The unsettled ongoing discussions on when a ratio is considered ‘elevated’ blurs the results obtained from NLR and PLR, and make comparisons of the studies challenging. Moreover, their binary outcome doesn’t allow a nuanced stratification into various risk groups.
To optimize the inflammation scores as prognostic biomarkers in cancer, combinations of the GPS and the NLR have been suggested as a more comprehensive reflection of the host inflammation. By combining the GPS and the NLR, five pro-inflammatory biomarkers were used in one scoring system (CNG) capturing various tumor-induced inflammatory reactions. This is demonstrated in gastric cancers [Citation19] and sarcomas [Citation20,Citation21] with promising results. The ACBS is another system that differs from the CNG in considering individual parameters and not ratios and in including serum hemoglobin level in the system. It is tested in both bone and soft tissue sarcomas. Until now, neither CNG nor ACBS has been evaluated in lung cancer patients.
Lung cancer remains heavily associated with smoking and it is therefore unsurprising that lung cancer patients commonly have other medical conditions (comorbidity). The presence of comorbidity affects the overall survival and may also be closely related to abnormal levels of several biomarkers [Citation22–24]. That is why, when evaluating inflammation as a prognostic marker it is of obvious importance to make adjustments for comorbidities to avoid erroneous conclusions. Unfortunately, so far, comorbidity has not been included in the evaluation of any of the inflammation-scores previously evaluated in lung cancer patients.
The primary objective of this prognostic cohort study was to evaluate the established inflammation-scores (NLR, PLR and GPS) as well as to compare the optimized inflammation-scores (CNG and ACBS) as prognostic markers in non-selected lung cancer patients taking the comorbidity into account. Furthermore, we hypothesized that the optimized inflammation-scores would improve the allocation of lung cancer patients.
Material and methods
Patients
In this cohort study, all patients referred to the Department of Pulmonary Medicine, Aarhus University Hospital, Denmark (n = 1.735) were included prospectively. The cohort has been studied in other contexts for studies on the epidermal growth factor system, exosome analyses and comorbidity evaluations. [Citation22,Citation25–27] In short, patients suspected of having lung cancer were included between April 2011 and September 2014. Patients with a final diagnosis of non-small cell lung cancer (n = 275) were used in this study. Patients with other cancer types (including SCLC) or without cancer were excluded, as were patients with no available pre-diagnostic blood sample. This results in a final cohort of lung cancer patients of 272. For detailed information on the inclusion and exclusion of the patients, see ref. [Citation26].
Data sources
The clinicopathological information (age, sex, histology, stage, PS, smoking and comorbidity) was acquired from the patients’ medical record at the time of inclusion and has been described in previous publications. [Citation26] The National Patient Registry (NPR) was used to obtain data on comorbidities prior to the lung cancer diagnosis. The NPR collects data on all admissions to Danish hospitals including dates of admission and discharge as well as discharge diagnoses for each patient. From the NPR, all discharge diagnoses between 1 January 1977 and the end of the study period (31 December 2016) were retrieved. The comorbidities were reported as Colinet Simplified Comorbidity Score (SCS), which includes renal insufficiency, other neoplastic disease, alcoholism, respiratory disease, cardiovascular disease and smoking [Citation28]. The vital status and cause of death was registered through linkage to the Central Population Registry and Cause of Death Registry.
Data on biomarkers was achieved from the clinical laboratory information system (LABKA) database. Test results were reported according to the international nomenclature, properties, and Units (NPU) coding system. Serum Albumin, CRP, hemoglobin (Hb), neutrophil count, lymphocytes count, and thrombocyte count were included in this study. The biomarkers were divided into normal or high/low, see .
Table 1. Inflammation markers.
Definition of inflammation-scores
GPS was computed on the basis of CRP and albumin. Patients were given the score as follows: 0 if albumin was high and CRP was low, 1 if either albumin was low or CRP was high and 2 if both albumin level was low and CRP level was high. A high neutrophil-to-lymphocyte ratio (NLR) was defined as values >5.0. The platelet-to-lymphocyte ratio (PLR) was defined as high if PLR >200. Two different optimized inflammation-scores were evaluated. The CNG (combination of NLR and GPS) [Citation19] was defined 0 if albumin was high and CRP was low and NLR >2. Patients with one, two or three abnormal test results were assigned a score of 0, 1, 2 or 3, respectively. The Aarhus composite biomarker score (ACBS) [Citation20] was defined as: 0 if albumin, CRP, neutrophils, lymphocytes, and hemoglobin were all within normal range, 1 if neutrophils were high, or lymphocytes were low or any other of the other mentioned biomarkers was abnormal, 2 if two biomarkers were abnormal and 3 if more than two biomarkers were abnormal.
Data analysis and statistics
Clinicopathological information, information from LABKA and NPR was linked on an individual level using the 10 digit civil personal registrations (CPR) number, which since 1968 has been assigned to all citizens in Denmark. The primary endpoints were overall survival. Using the Cox proportional hazard model crude and adjusted analyses were estimated. The following variables were included in the adjusted analysis: age (continuous variable), comorbidity (yes vs. no), histological type (squamous and non-squamous), stage (I/II/IIIa vs IIIb/IV), and performance status (PS0/1 vs PS2/3). Cumulative incidences were used to visualize the impact of each biomarker scoring system, these analyses were adjusted for age and comorbidity. Survival analysis on the effect of each biomarker scoring system according to stage was performed using the Kaplan–Meier method. To evaluate whether or not the inflammation-scores would add prognostic value to the well-established TNM and PS, the models were compared by the Akaike's information criteria (AIC) and by Harrell's concordance index (C-index). For AIC the values are arbitrary and the model with the minimum AIC is the model with the optimal fit of data. Only a difference of 2 or more is considered a true difference. For the C-index, values range between 0.5–1.0 where 1.0 is the perfect fit. All statistical tests were performed by Stata version 14. A two-sided p value less than .05 was regarded significant.
Ethics
Before inclusion, all patients gave informed written consent. The study was approved by the Central Denmark Region Committees on Biomedical Research Ethics (M-20100246) and the Danish Agency of Data Protection (1-16-02-346-14).
Results
Patient characteristics
The median age at diagnosis was 68 years (range 39–89). Comorbidity was present in 61% of the patients. Clinicopathological characteristics are described in . At the time of follow-up 202 of the 275 patients had died. The median follow-up time was 1.4 years, and for patients still alive the median follow-up time was 3.4 years.
Table 2. Patient characteristics.
Biomarkers
Each biomarker was investigated separately. Low albumin was present in 83 (31%) of the patients, high CRP in 125 (46%), low hemoglobin in 73 (27%), high neutrophil counts in 87 (32%) of the patients, low lymphocytes in 66 (24%), and high platelets in 60 (22%) of the patients. Each biomarker is described in .
The crude survival analyses are seen in Supplementary Table 1. Based on the crude analysis, all the biomarkers were potential prognostic biomarkers for overall survival.
Inflammation-scores
Based on the individual biomarkers, the GPS, NLR, PLR CNG and ACBS were composed.
The crude and adjusted survival analysis for the GPS, NLR, PLR, CNG and ACBS are shown in , and survival-curves depicted as cumulative incidence are shown in . For all inflammation-scores, a higher score was associated with worse prognosis. When adjusting for confounders of the association between each scoring system and overall survival, each inflammation-score remained significant as an independent prognostic factor of inferior survival. However, as is demonstrated in , only when using the optimized inflammation-scores CNG and ABCS is the distribution of patients between risk groups became even-numbered. To compare the inflammation-scores, AIC and C-index was calculated for each individual inflammation-score (). Based on these findings, the optimized inflammation-scores CNG and ACBS are the models best predicting the survival. To evaluate if the inflammation-scores add value to the most important and well-established prognostic markers in oncology TNM and PS, AIC and C-index were calculated with or without these inflammation-scores (). This demonstrates that each inflammation-score adds value to the TNM and PS, though; the inflammation-score with the best improvement is the ACBS.
Figure 1. Survival-curves for each inflammation-score demonstrated by the cumulative incidence in (a) ACBS, (b) CNG (c) GPS, (d) NLR, and (e) PLR. For each inflammation-score, a higher score is associated with worse prognosis. The distribution of patients into the subgroups is optimized in the ACBS and CNG compared to the NLR, PLR and GPS. ACBS: Aarhus composite biomarker score; NLR: Netrophile-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; GPS: Glasgow prognostic score; CNG: Combination of NLR and GPS.
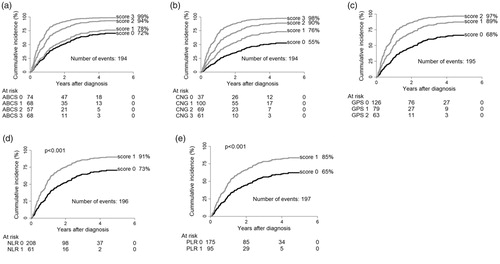
Table 3. Survival analysis - inflammation-scores.
Table 4. Predictive accuracies of the prognostic models.
Inflammation-scores by stage
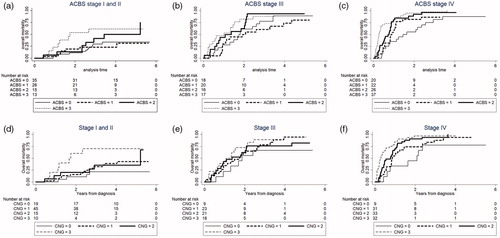
The inflammation-scores were then evaluated within stages and overall mortality curves were made ( and Supplementary Figure 1). For the low stages I and II, all scores except PLR were able to identify patients with a poor prognosis ( and Supplementary Figure 1). For the advanced stage IV separation of patients into high-risk and low-risk patients was not as pronounced as for stage I and II. For the stage III patients, none of the inflammation-scores were able to identify patients with inferior survival.
Figure 2. Survival-curves for the optimized inflammation-curves demonstrated by cumulative incidence in ACBS and CNG. For each inflammation-score, the patients have been split based on stage. Identification of patients with the worst survival is clear especially in stage I and II patients. No separation is observed in stage III patients. (a) ACBS in stage I and II, (b) ACBS in stage III, (c) ACBS in stage IV, (d) CNG in stage I and II, (e) CNG in stage III and (f) CNG in stage IV. ACBS: Aarhus composite biomarker score; CNG: Combination of neutrophil-to-lymphocyte ratio and Glasgow prognostic score.
Discussion
In this prospective cohort study of 275 NSCLC patients, we found that high levels of inflammation-scores are prognostic markers of inferior survival. Furthermore, we demonstrated that the allocation of patients is greatly improved when using the optimized inflammation-scores – CNG and ACBS. With smoking being the leading cause of lung cancer, comorbidity and inflammation are commonly present. Due to the high quality of the Danish public registers [Citation29,Citation30], as the first, we were able to evaluate and compare inflammation-scores in lung cancer patients taking comorbidity into account. The inflammation-scores retained their prognostic ability even after correction for comorbidities. These results suggest that these inflammation-scores reflect a cancer related mechanisms and they may merit being integrated into lung cancer treatment algorithms. In the study, we included patients independently of stage and histological subtypes as a reflection of the inhomogeneous lung cancer population. By doing so, we could test the true value of this potential prognostic marker in an everyday clinical setting.
For new prognostic markers to be accepted, they have to be accessible, reproducible, and give extra information to the clinician. The inflammation-scores are built on combinations of CRP, albumin, neutrophil counts, lymphocytes, platelets or hemoglobin, which are all part of routine diagnostic work-up. This gives the inflammation-scores great potential for being applied. Furthermore, in low-income countries, where lung cancer incidence is still rising, this could be a simple, inexpensive additional tool in managing lung cancer patients.
Comparisons of the studies on GPS, NLR and PLR is challenging since cut-points defining elevated levels is an ongoing discussion. Nevertheless, meta-analyses have been made, demonstrating that high levels of NLR and PLR are prognostic markers of inferior survival [Citation31]. As opposed to most studies on NLR and PLR, we applied a predefined cut-point of NLR ≥5 and PLR >200, which is a conservative approach.
In studies on NLR, PLR and GPS, patients are dived into two or three groups. By optimizing the allocation of patients, the clinician can differentiate the management of the patient at the individual level. Therefore, the new optimized inflammation-scores CNG and ACBS were developed to optimize the allocation of patients. This has previously been demonstrated in gastric cancer [Citation19] and in sarcomas [Citation20,Citation21] In this study, we demonstrate that the CNG and ACBS are able to allocate patients into even-numbered risk groups. The CNG and ACBS are very similar; however, there are minor differences. Firstly, the ACBS includes hemoglobin. Secondly, the ACBS evaluates each marker individually and do not include any ratios. When using a ratio, an inborn error is accepted since the ratio can be of equal number independently of the actual level of the given marker. Still, the CNG was not inferior to the ACBS in this study, though, when combining the inflammation-scores with the TNM and PS, the ACBS is the inflammation-score that adds most value to the existing prognostic markers.
An important point to consider when contemplating a new prognostic marker is what extra information it gives to the clinician. In the impressive study by Yotsukura et al. [Citation18], GPS was evaluated in 1048 patients with stage I or II NSCLC. They demonstrate that in the group of patients with the best prognosis based on the TNM, the GPS is able to identify patients with an inferior survival. This is important information that may have direct implication on decision-making. In this study, we demonstrate in the early stage patients, that all the evaluated inflammation-scores, except PLR, were able to identify patients with an inferior survival. These findings need to be validated in a larger study. Still, the perspectives are interesting, could the inflammation-scores be a tool for deciding how close – or aggressive – we should monitor and treat the patients?
There has not yet been any comprehensive hypothesis about the biological mechanisms that these various inflammation-scores reflect that could explain why and how these biological mechanisms are associated with inferior survival. In Rheumatic disease, high interleukin-1β levels are observed along with anemia, neutrophilia, lymphopenia, low albumin and elevated CRP. [Citation32] From animal models, interleukin-1β is known to be associated with cancer invasiveness, growth, and metastasis. [Citation33] Recently, Ridker et al. demonstrated that inhibition of interleukin-1β is associated with lower lung cancer incidence as well as mortality compared to placebo in patients with atherosclerosis. [Citation34] Our results may suggest that elevated IL-1β is the driving mechanism behind the abnormal serum biomarkers. The prognostic value of these serum biomarkers in various cancer types points to a universal cancer-driven inflammatory mechanism that may involve interleukin-1β, giving this observation a far reaching clinical implication. In a future prospective study, we plan to evaluate the interleukin-1β level as well as inhibition of interleukin-1β in various cancer types.
In conclusion, in a prospective cohort study we have demonstrated that inflammation-scores are prognostic markers of inferior survival in unselected lung cancer patients even when comorbidity was taken into account. Furthermore, we demonstrated that the optimized inflammation-scores improved the allocation of patients regardless of the clinicopathological characteristics and could detect a subgroup of patients in the early stages that harbor a worse prognosis than expected.
Supplemental Material
Download Zip (257.1 KB)Disclosure statement
The authors report no conflicts of interest.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics. CA Cancer J Clin. 2014;64:9–29.
- The Danish National Lung Cancer Group “Årsrapport 2014.” 2014. Available at: http://lungecancer.dk/documents/ED76D14E-3915-4D9C-9033-55D1B03402FB.pdf.
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours, 7th Edition. Chichester, West Sussex, UK: Wiley-Blackwell. 2009. Available at: http://eu.wiley.com/WileyCDA/WileyTitle/productCd-1444332414.html. Accessed March 29, 2016.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7165009. Accessed November 11, 2017.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674.
- McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–540.
- Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48:155–170.
- Wang D, Luo H, Qiu M, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012;29:3092–3100.
- Lorente D, Mateo J, Templeton AJ, et al. Baseline neutrophil-lymphocyte ratio (NLR) is associated with survival and response to treatment with second-line chemotherapy for advanced prostate cancer independent of baseline steroid use. Ann Oncol off J Eur Soc Med Oncol. 2015;26:750–755.
- Koh C-H, Bhoo-Pathy N, Ng K-L, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015;113:150–158.
- Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47:2633–2641.
- Shiba H, Misawa T, Fujiwara Y, et al. Glasgow Prognostic Score predicts outcome after surgical resection of gallbladder cancer. World J Surg. 2015;39:753–758.
- Hwang J-E, Kim H-N, Kim D-E, et al. Prognostic significance of a systemic inflammatory response in patients receiving first-line palliative chemotherapy for recurred or metastatic gastric cancer. BMC Cancer. 2011;11:489.
- Wang D, Ren C, Qiu M, et al. Comparison of the prognostic value of various preoperative inflammation-based factors in patients with stage III gastric cancer. Tumor Biol. 2012;33:749–756.
- Zhang H, Zhang L, Zhu K, et al. Prognostic significance of combination of preoperative platelet count and neutrophil-lymphocyte ratio (COP-NLR) in patients with non-small cell lung cancer: based on a large cohort study. PLoS One. 2015;10:e0126496.
- Pinato DJ, Shiner RJ, Seckl MJ, et al. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br J Cancer. 2014;110:1930–1935.
- Zhu L, Chen S, Ma S, et al. Glasgow prognostic score predicts prognosis of non-small cell lung cancer: a meta-analysis. Springerplus. 2016;5:439.
- Yotsukura M, Ohtsuka T, Kaseda K, et al. Value of the Glasgow Prognostic Score as a prognostic factor in resectable non-small cell lung cancer. J Thorac Oncol. 2016;11:1311–1318.
- Liu X, Chen S, Liu J, et al. Impact of systemic inflammation on gastric cancer outcomes. Coleman WB, ed. PLoS One. 2017;12:e0174085.
- Aggerholm-Pedersen N, Maretty-Kongstad K, Keller J, et al. The prognostic value of serum biomarkers in localized bone sarcoma. Transl Oncol. 2016;9:322–328.
- Maretty-Kongstad K, Aggerholm-Pedersen N, Keller J, et al. A validated prognostic biomarker score for adult patients with nonmetastatic soft tissue sarcomas of the trunk and extremities. Transl Oncol. 2017;10:942–948.
- Sandfeld-Paulsen B, Meldgaard P, Aggerholm-Pedersen N. Comorbidity in lung cancer: a prospective cohort study of self-reported versus register-based comorbidity. J Thorac Oncol 2018;13(1):54–62.
- Deleuran T, Thomsen RW, Nørgaard M, et al. Comorbidity and survival of Danish lung cancer patients from 2000–2011: a population-based cohort study. Clin Epidemiol. 2013;5:31–38.
- Iachina M, Jakobsen E, Møller H, et al. The effect of different comorbidities on survival of non-small cells lung cancer patients. Lung. 2015;193:291–297.
- Sandfeld-Paulsen B, Folkersen BH, Rasmussen TR, et al. Gene expression of the egf system—a prognostic model in non–small cell lung cancer patients without activating EGFR mutations. Transl Oncol 2016;9:306–312.
- Sandfeld-Paulsen B, Jakobsen KR, Bæk R, et al. Exosomal proteins as diagnostic biomarkers in lung cancer. J Thorac Oncol. 2016;11:1701–1710.
- Sandfeld-Paulsen B, Aggerholm-Pedersen N, Bæk R, et al. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol Oncol 2016;10:1595–1602.
- Colinet B, Jacot W, Bertrand D, et al. A new simplified comorbidity score as a prognostic factor in non-small-cell lung cancer patients: description and comparison with the Charlson’s index. Br J Cancer 2005;93:1098–1105.
- Søgaard M, Kornum JB, Schønheyder HC, et al. Positive predictive value of the ICD-10 hospital diagnosis of pleural empyema in the Danish national registry of patients. Clin Epidemiol. 2011;3:85–89.
- Thomsen RW, Lange P, Hellquist B, et al. Validity and underrecording of diagnosis of COPD in the Danish National Patient Registry. Respir Med 2011;105:1063–1068.
- Peng B, Wang Y-H, Liu Y-M, et al. Prognostic significance of the neutrophil to lymphocyte ratio in patients with non-small cell lung cancer: a systemic review and meta-analysis. Int J Clin Exp Med. 2015;8:3098–3106. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26064198. Accessed November 11, 2017.
- Harrison P, Pointon JJ, Chapman K, et al. Interleukin-1 promoter region polymorphism role in rheumatoid arthritis: a meta-analysis of IL-1B-511A/G variant reveals association with rheumatoid arthritis. Rheumatology. 2008;47:1768–1770.
- Apte RN, Dotan S, Elkabets M, et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408.
- Ridker PM, MacFadyen JG, Thuren T, et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–1842.
