Abstract
Purpose: To validate a normal tissue complication probability (NTCP) model for late unfavourable aesthetic outcome (AO) after breast-conserving therapy.
Materials/Methods: The BCCT.core software evaluated the AO using standardized photographs of patients treated at the University Hospitals Leuven between April 2015 and April 2016. Dose maps in 2 Gy equivalents were calculated assuming α/β = 3.6 Gy. The discriminating ability of the model was described by the AUC of the receiver operating characteristic curve. A 95% confidence interval (CI) of AUC was calculated using 10,000 bootstrap replications. Calibration was evaluated with the calibration plot and Nagelkerke R2. Patients with unfavourable AO at baseline were excluded.
Patient, tumour and treatment characteristics were compared between the development and the validation cohort. The prognostic value of the characteristics in the validation cohort was further evaluated in univariable and multivariable analysis.
Results: Out of 175 included patients, 166 were evaluated two years after RT and 44 (26.51%) had unfavourable AO. AUC was 0.66 (95% CI 0.56; 0.76). Calibration was moderate with small overestimations at higher risk. When applying all of the univariable significant clinicopathological and dosimetrical variables from the validation cohort in a multivariable model, the presence of a seroma and V45 were selected as significant risk factors for unfavourable AO (Odds Ratio 4.40 (95% CI 1.96; 9.86) and 1.14 (95% CI 1.03; 1.27), p-value <.001 and .01, respectively).
Conclusions: The NTCP model for unfavourable AO shows a moderate discrimination and calibration in the present prospective validation cohort with a small overestimation in the high risk patients.
Introduction
Breast cancer is the most common malignancy in women worldwide [Citation1]. Breast-conserving therapy (BCT), including breast-conserving surgery followed by radiotherapy (RT) is the standard for locoregional care since level-I evidence has shown its equivalence with mastectomy in terms of survival [Citation2–4]. With improvements in screening, diagnosis and therapy, the oncological outcome of breast cancer patients has improved in the last decades, resulting in a growing number of long-term survivors [Citation5]. Consequently, the long-term toxicity of treatment and its impact on quality of life are of paramount importance [Citation6]. For RT, long-term cardiac and lung toxicity as well as the aesthetic outcome (AO) are of main interest.
BCT aims for preservation of the intact breast with satisfactory AO. Long-term unfavourable AO presents in around one third of the patients, however different assessments methods result in a wide range of outcomes [Citation7,Citation8]. Several patient-, tumour- and treatment-related factors contribute to the AO [Citation7,Citation9,Citation10]. In clinical practice, predictive algorithms can be used to inform patients about the treatment and expected outcomes and to guide decision-making. Furthermore, a predictive nomogram can be used to improve treatment towards a patient-tailored approach. So far, however, validated prognostic models on the AO after BCT are lacking. Ultimately, by taking clinicopathological and dose-volume metrics into account in a prediction model (a so called normal tissue complication probability model, NTCP), the dose distribution of individual treatment plans could be optimized and patients could be informed on the risk of late unfavourable AO.
Recently, a prediction model for late unfavourable AO has been developed taking into account clinicopathological variables and dose-volume metrics [Citation11]. An objective and thorough validation of an NTCP model is mandatory before its widespread implementation as a useful clinical tool. The primary aim of the present study was to evaluate the NTCP model by using an independent, prospectively collected patient cohort.
Material & methods
The TRIPOD (Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis) recommendations for the validation of a prediction model were followed [Citation12]. The study was approved by the Clinical Trial Centre and the Ethical Committee of our institution.
Patient cohort
The population of this prospective cohort study was composed inviting all consecutive patients treated with BCT for breast cancer with curative intent who consulted the RT department at the University Hospitals Leuven, Belgium between April 2015 and April 2016. Informed consent was obtained. The sample size was calculated to independently estimate AO in two treatment groups based on the technique used to irradiate the tumour bed after whole-breast irradiation. In both the brachytherapy boost and the external beam radiotherapy boost group a 25% unfavourable AO measured with BCCT.core was assumed after 2 years. Calculations were performed for estimating with 80% power the 95% confidence interval (CI) with a halfwidth of 10%. For each group 76 patients were needed [Citation13,Citation14].
Aesthetic outcome
Patients were evaluated at two pre-set time points: after surgery and before the start of RT; and 2 years after RT.
An anterior view picture of the patient was taken with the patients hands on the hips. The BCCT.core software was used to evaluate the AO [Citation8,Citation15]. Based on the semi-automatic localization of fiducial points (nipple complex, breast contour, sternal jugular notch, mark 25 cm downwards from the jugular notch), the software firstly measures asymmetry, skin colour changes and surgical scar appearance. Secondly, the set of measures is automatically converted into an overall objective AO: excellent, good, fair or poor [Citation15]. AO was dichotomized in excellent/good (‘favourable’) versus fair/poor (‘unfavourable’).
Treatment
BCT included whole-breast irradiation followed by a boost to the tumour bed in all patients. The whole breast was irradiated with two tangential fields and field-in-field RT. Two fractionation schedules were applied for whole-breast irradiation: normofractionation (50 Gray (Gy) in 25 fractions) and hypofractionation (42.56 Gy in 16 fractions). The calculation model used, was AAA from Eclipse (Varian Medical Systems, Palo Alto, CA). For the boost, either 16 Gy in 8 fractions or 13.3 Gy in 5 fractions was applied with electrons or photons, or a high dose rate brachytherapy with a dose of 8.5 Gy. For the selection of the boost technique, an in-house developed flowchart based on the depth of the tumour bed was used. For a clinical target volume lying more than 28 mm beneath the epidermis, an interstitial boost was chosen over an electron boost because of skin doses. If the patients refused a brachytherapy boost, or if it was technically impossible, a photon boost was suggested [Citation16]. Treatment regimens were discussed at the multidisciplinary tumour board. Regional nodal irradiation was allowed. Hormonal therapy included tamoxifen and aromatase-inhibitors in different regimens and was prescribed in hormone receptor-positive breast cancer patients. Chemotherapy was given according to standard protocol and included epirubicin, cyclophosphamide and taxanes.
Data collection
Patient-, tumour-, and treatment-related characteristics were prospectively collected. Dose-volume metrics for the breast were assessed from the individual patient RT plans. The breast volume was delineated according to the guidelines from the EORTC, however also included the 5 mm of breast skin [Citation17]. Summed dose maps in 2 Gy equivalents (EQD2) were calculated assuming α/β = 3.6 Gy [Citation18]. In case of a brachytherapy boost, a Deformable Image Registration mapped the brachytherapy CT to the external beam planning CT in MIM 6.1.7 (MIM software, Cleveland, OH) using a free-form intensity-based registration algorithm. After a warping of the brachytherapy dose map, a dose summation was performed. For the logistic regression analysis, the considered dose-volume metrics included the relative volumes of the breast receiving 5 Gy (V5) to 70 Gy (V70) in steps of 5 Gy and the mean breast dose.
NTCP model
The primary aim of the study was to validate the following NTCP model for late unfavourable AO: NTCP =1/(1 + e−t), where t = −2.6759+(0.0574 × V55) + 1.5546 (if seroma + 1.2029 (if ALND) (ALND = axillary lymph node dissection) [Citation11]. The NTCP model was developed based on 121 retrospectively evaluated patients treated between two and six years ago of whom 44 (36%) developed unfavourable AO. The model performed well with an Area Under the Curve (AUC) of the receiver operating curve 0.76 and bootstrap resampling resulting in AUC 0.75. The Hosmer–Lemeshow test assessing calibration was not significant, indicating good agreement between expected and observed unfavourable AO rates [Citation11].
Validation
There are two aspects in the evaluation of an NTCP model: discrimination and calibration. Discrimination is the extent to which patients predicted to be at higher risk of unfavourable AO exhibit higher event rates compared to those at lower risk. The discriminating ability of the model was described by the AUC of the receiver operating characteristic curve [Citation19]. A 95% confidence interval (CI) of AUC was calculated using 10,000 bootstrap replications. A model with perfect discrimination would have an AUC of 1, whereas a value of 0.5 indicates a coin toss would provide information as accurate as that given by the model. Calibration reflects the prediction accuracy, or the absence of over- or underestimation of the actual risk of unfavourable AO. Calibration was evaluated with the calibration slope, calibration-in-the-large and the Nagelkerke R2 (a pseudo R-squared metric indicating the goodness-of-fit) [Citation20]. The calibration curve for the model validation was adapted in order to bin the predicted probabilities using the same bins (same boundaries on predicted probability axis) as for the model training calibration curve. This offers a better comparison of the curves for training and validation. Patients with unfavourable AO at baseline were excluded for the validation.
Building a new model
The second aim of the present study was to build a new model. The TRIPOD recommendations for a type 1B study were followed [Citation12]. Model performance was assessed with the area under the curve (AUC) of the receiver operating characteristic curve and bootstrap sampling, with the Hosmer–Lemeshow test and the calibration plot. Patient, tumour and treatment characteristics were compared between the development and the validation cohort. The Mann–Whitney test was used for continuous and ordinal variables, the Fisher’s Exact test for proportions. The prognostic value of the characteristics was further evaluated in univariable and multivariable analysis. A 100-times three-fold cross validation was performed to assess the robustness of the dose-volume metrics. A Spearman correlation r of all variables with p < .1 at univariable analysis was performed. For variables with r > 0.7, the variable with the lowest p value was retained. The remaining variables were then included in the stepwise forward logistic regression. A multivariable forward stepwise model was built with covariate inclusion at p-value<.05 and removal at p ≥ .05. All analyses have been performed using Statistica (version 13, Dell Inc, Tulsa, USA).
Results
Four patients refused participation and 175 patients were included in the present validation cohort. No photographic evaluation was available for five patients two years after RT. One patient underwent bilateral mastectomy between one and two years after RT. Three patients out of the 175 (1.71%) had unfavourable AO at baseline and the outcome did not improve two years after RT. Eighty patients had received a brachytherapy boost and 95 an external beam RT boost. Of the 166 patients evaluated two years after RT, 44 (26.51%) had unfavourable AO. lists the comparisons between the development and the validation cohort. Overall, patients in the validation cohort had significantly smaller breasts (mean volume 742.2 cc vs 902.8 cc), a lower body-mass index (25.3 kg/m2 vs 26.8 kg/m2), were more often treated with hypofractionated radiotherapy (60.0% vs 30.6%) and underwent less ALND (13.1% vs 24.0%).
Table 1. Comparison of patient, tumor and treatment characteristics in the development and validation cohort.
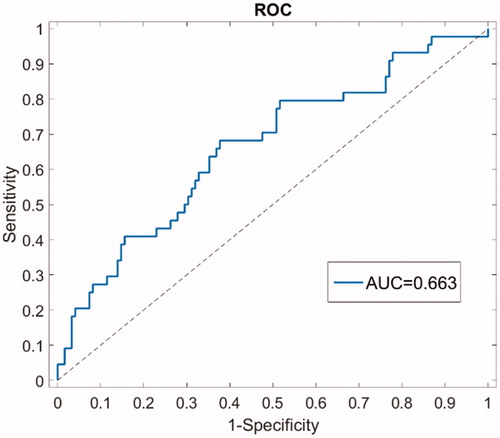
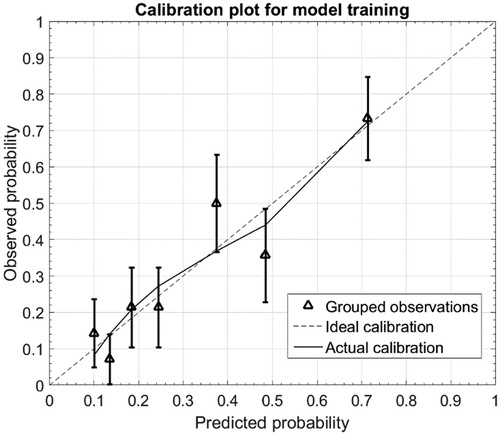
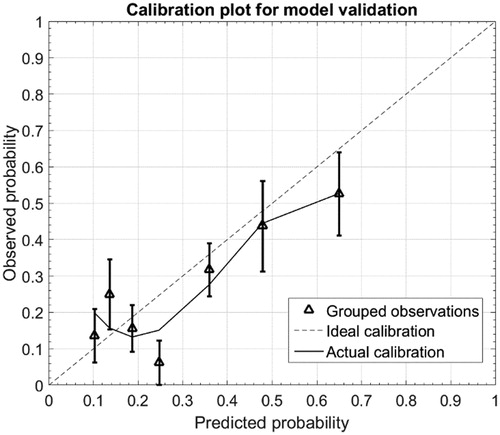
AUC was 0.66 (95% CI 0.56; 0.76), indicating moderate discriminative ability of the NTCP model in the present cohort () (). and show the calibration plot for the development cohort and for the validation cohort, respectively. In the development cohort, the calibration slope was 0.98 close to the ideal slope of 1, and calibration-in-the-large (intersection of linear fit with y-axis) was 0.006, close to the ideal intersection at 0. As correlation coefficient, the Nagelkerke R2 was 0.27. For the validation cohort, the calibration slope was 0.74 and calibration-in-the-large was 0.042. The Nagelkerke R2 was 0.13. The outlier fourth data point on the predicted probability axis corresponds to a bin with only 16 patients available for validation (for comparison, the next data point combines data from 41 patients).
Table 2. Performance of the NTCP model.
In univariable analysis a larger breast band size, body-mass index, surgical specimen weight, volume of the breast and the presence of a seroma were significantly correlated with unfavourable AO (). The relative volumes of breast tissue from V30 to V45 were also significantly associated with unfavourable AO. In contrast to the development cohort, chemotherapy, an ALND, the mean dose to the breast and volumes receiving higher doses (V55-V70) were not withheld. Follow-up times in the development set varied between 2.1 years and 6.0 years. Univariable logistic regression of unfavourable AO with follow-up time as continuous variable was not significant, p = .13 and Odds Ratio (OR) 1.37 (95% CI 0.92; 2.04). However, when limiting the development dataset to only the lowest quartile follow-up times shorter than 2.75 years (24 patients, 6 events), univariable logistic regression resulted in a non-significant covariate V55 (OR 1.07, p = .19), while the covariate V45 was significant (OR 1.50, p = .05). A Spearman correlation r of all variables with p < .1 at univariable analysis (13 variables) was now performed. For variables with r > .7, the variable with the lowest p value was retained. This way, V30, V35 and V40 were excluded for their high correlation with V45 (r > 0.9), while volume of the breast was excluded for correlation with BMI (r = 0.78). The nine remaining variables were then included in the stepwise forward logistic regression. The final model included V45 and the presence of a seroma as significant risk factors for unfavourable AO (OR 4.40 (95% CI 1.96; 9.86) and 1.14 (95% CI 1.03; 1.27), p-value<.001 and .01, respectively). Beta-estimates were −12.68 for b0, 0.13 for V45 and 1.48 for seroma. AUC was 0.75 and bootstrap sampling resulted in AUC 0.75 (95% CI 0.65; 0.83) (). The calibration slope was 1.02 and calibration-in-the-large was -0.01. The Nagelkerke R2 was 0.17 (). The Hosmer–Lemeshow test was not significant (Chi square 6.94, p = .54) indicating good agreement between expected and observed unfavourable AO rates.
Table 3. Univariable analysis for unfavourable aesthetic outcome in development and validation cohort.
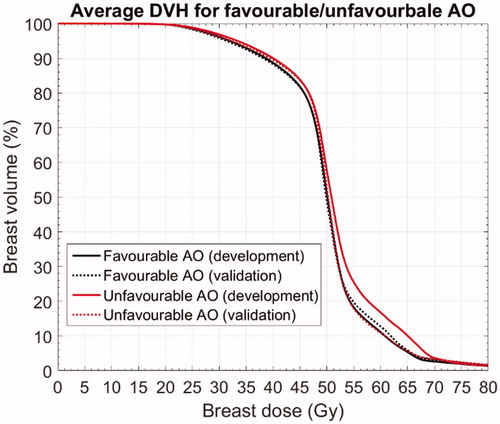
shows the average breast dose-volume histograms (DVH) for favourable and unfavourable aesthetic outcome in the development and validation cohort. The higher V45 associated with unfavourable AO observed in the development cohort was perfectly reproduced in the validation cohort (identical behaviour of solid (development) and dotted (validation) lines in the dose range below 50 Gy). On the other hand, the large difference in V55 (and higher doses up to V65) between favourable and unfavourable AO observed in the development cohort (solid lines) was not reproduced in the validation cohort (dotted lines).
Discussion
More than 75 percent of the early stage breast cancer patients survive long term, making treatment-induced side-effects important outcome measures. Currently, aesthetic outcome (AO) is becoming increasingly important because it influences the quality of life and psychosocial recovery after breast cancer treatment. An NTCP model to predict for the risk of long-term AO has previously been developed by our research group [Citation11]. The nomogram included three variables: seroma, ALND and V55. Resampling using 10,000 bootstrap replications, resulted in an AUC of 0.75 (95% CI 0.64;0.85) and good calibration was achieved. The primary purpose of the present study was to externally validate the predictive model to determine its performance when applied to an independent dataset.
A prospective study was therefore set up inviting all consecutive patients treated with BCT at the University Hospitals Leuven between April 2015 and April 2016. The cohort included 175 patients of whom 166 patients were included in the analysis two years after treatment. The high rate of assessments at two years was a strength of the present study. Aesthetic outcome was unfavourable in three out of 175 patients (1.71%) at baseline and in 44 out of the 166 patients (26.51%) two years after RT. Concerning performance of the model in the present cohort, the AUC was 0.66 (95% CI 0.56; 0.76), indicating moderate discriminative ability of the NTCP model. Calibration was moderate with overestimations for the risk in the highest risk zone.
Several factors contribute to the lower discriminative ability of the model in the validation cohort. Firstly, concerning the variables included in the NTCP model, it is important to notice that in the developmental cohort, ALND and seroma were most decisive for unfavourable AO and that V55 had a smaller effect. In the validation cohort, seroma seemed even more important (OR 4.71 compared to 2.80, p = .02), ALND to a lesser extent and anyhow not significant (OR 2.25 compared to 3.17, p = .06) and V55 had no effect in univariable analyses. V45 however was now the most significant dose-volume metric and also 100-times three-fold cross validation confirmed the robustness of V45 (variable was selected in 91% of the folds). In multivariable analysis, only seroma and V45 were withheld in the present validation cohort. In the development cohort, V45 also had a significant effect, albeit less than V55. However, V45 and V55 were not correlated in the validation cohort (p = .85).
Secondly, testing the difference between the development and the validation cohort indicated that patients in the latter had smaller breasts, lower body-mass indices, were more often treated with hypofractionation and received less ALND. With only 13% of the patients (n = 23) that underwent an ALND in the validation cohort compared to 24% in the development cohort (n = 29), there is more uncertainty on the impact of the ALND which might be a cause of the decreased discriminative ability.
Thirdly, no baseline photographs were available for the development cohort whereas 3 patients were excluded because of unfavourable AO at baseline in the validation cohort.
Fourthly, patients were evaluated two years after RT in the validation cohort and the median follow-up in the development cohort was 3.4 years. The event rates were also lower: 27% versus 36%. A possible explanation would be that AO deteriorates with time, but this is in contrast to the data of Hennigs et al who have shown there was no measurable change in AO measured by BCCT.core between two and six years after surgery [Citation7].
Combining the information on the dose-volume metrics V45 and V55 with the follow-up time and event rates, leads us to the following hypothesis. The volume effect of the size of the breasts might be related to AO two years after RT and the dose-volume effect of RT might show later.
Firstly, larger breasts (as described by breast volume, body mass index or band size) are known to be correlated with an unfavourable AO and also in the present cohort, these variables were significant [Citation21]. V45 associates well with the volume effect of the breast size. V45 of the breast is higher in larger breasts due to the lower contribution of the low V45 in the skin build-up region compared to smaller breasts. In smaller breasts, the skin and thus build-up region form a relative higher volume of the breast tissue and thus V45 values are lower. V45 is thereby related to AO two years after RT.
Secondly, the V55 included in the NTCP model might then relate to unfavourable AO later than two years after RT. With prescription doses to the whole-breast of EQD2 50 Gy and to the boost of EQD2 66 Gy, V55 relates to the boost dose.
Our hypothesis is supported by the fact that V45 and V55 were not correlated and thus can be regarded as two different sources of information: V45 for breast volume and V55 for boost volume. Additionally, in a subgroup of the large EORTC boost vs no boost trial, breast fibrosis (which is a part of total AO) was also related to the administration of the boost dose three years after treatment [Citation22]. Furthermore comparisons between the BT group and the EBRT group add to the hypothesis. In the development cohort complication rates for both modalities were identical (OR 1.07, p-value .86). In the validation cohort however, higher unfavourable AO rates were observed in the BT group compared to the EBRT (OR 1.96, p-value .06). Breast volumes in the validation cohort were larger with BT (899.4 cc versus 615.6 cc, p = .001), reflecting the effect of V45. As in the boost vs no boost trial, boost volumes in our analysis seemed larger in the EBRT boost compared to the BT boost (159.6cc versus 104.2 cc, p < .0001) [Citation23]. When limiting the development dataset to only the lowest quartile follow-up times shorter than 2.75 years, univariable logistic regression resulted in a non-significant covariate V55 (OR 1.07, p = .19), while the covariate V45 was significant (OR 1.50, p = .05). These are similar results to what was observed in the validation set where all follow-up times are around 2 years and thus corroborates our hypothesis of the breast volume (correlated to V45) impact detected in the analysis around 2 years, while the dosimetric effect on outcome probably only occurs at a later time. It might thus be that the unfavourable AO related to V55 might still become apparent in the present EBRT group with time. This would again result in equal complication rates in both boost technique groups. Continued follow-up of the present cohort is foreseen up to five years after treatment, where the hypothesis that the boost effect (V55) occurs later as the effect of the volume of the breast (V45) will be further addressed.
Added to that, further research is necessary specifying the implication of other variables such as volumetric breast density calculated on mammography which has been shown to correlate with unfavourable long-term AO measured by BCCT.core [Citation24]. Tests for identification of patients with increased risk of radiation-induced subcutaneous fibrosis are also being investigated and some are even validated, such as single nucleotide polymorphisms and gene expression analysis [Citation25–30].
The secondary aim of the present study was to develop a new NTCP model. The optimal new model included V45 and seroma and the following formula is proposed: NTCP =1/(1 + e−t), where t= −12.68+(0.013 × V45)+1.48 (if seroma). Independent and external validation of this new model is necessary before clinical implementation. Furthermore, several limitations must be considered [Citation11]. The deformable registration of the brachytherapy CT scan with the external beam planning CT scan for whole breast irradiation might lead to inaccuracies. This deformation of the breast however is inherent to the brachytherapeutic technique with the use of interstitial needles. Also, all patients were treated with a boost to the tumour bed. Extrapolation of these data to higher or lower doses for V55 or V45 as in the present study should be tested before clinical use.
In conclusion, the results of our study have demonstrated that the NTCP model for unfavourable AO shows a moderate discrimination and calibration in the present prospective validation cohort with a small overestimation in the high risk patients.
Disclosure statement
The authors report no conflicts of interest.
References
- Ferlay J, Soerjomataram I, Ervik M, et al.GLOBOCAN 2012 v1.1, Cancer Incidence and [Online]. Lyon, France: International Agency for Research on Cancer; 2014. [Cited: March 25, 2018.] Available at: http://globocan.iarc.fr
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241.
- Litière S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol. 2012;13:412–419.
- Senkus E, Kyriakides S, Ohno S, on behalf of the ESMO Guidelines Committee, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v8–30.
- Desantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62.
- Ewertz M, Jensen A. Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncol. 2011;50:187–193.
- Hennigs A, Hartmann B, Rauch G, et al. Long-term objective cosmetic outcome after breast-conserving therapy. Breast Cancer Res Treat. 2015;153:345–351.
- Cardoso MJ, Cardoso JS, Vrieling C, et al. Recommendations for the aesthetic evaluation of breast cancer conservative treatment. Breast Cancer Res Treat. 2012;135:629–637.
- Vrieling C, Collette L, Fourquet A, et al. The influence of patient, tumor and treatment factors on the cosmetic results after breast-conserving therapy in the EORTC 'boost vs. no boost' trial. EORTC Radiotherapy and Breast Cancer Cooperative Groups . Radiother Oncol. 2000;55:219–232.
- Santos G, Urban C, Edelweiss M, et al. Long-term comparison of aesthetical outcomes after oncoplastic surgery and lumpectomy in breast cancer patients. Ann Surg Oncol. 2015;22:2500–2508.
- Kindts I, Defraene G, Laenen A, et al. Development of a normal tissue complication probability model for late unfavourable aesthetic outcome after breast-conserving therapy. Acta Oncol. 2018;13:1–8. [Epub ahead of print]
- Collins G, Reitsma J, Altman D, et al. Transparant reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Br J Cancer. 2015;12:251–259.
- Chow S, Shao J, Wang H. (2008) Sample size calculations in clinical research, 2nd Ed. s.l. edn. Boca Raton, FL, USA: Chapman & Hall/CRC (Biostatistics Series)
- Kindts I, Laenen A, Christiaens M, et al. Comparison of brachytherapy and external beam radiotherapy boost in breast-conserving therapy: patient-reported outcome measures and aesthetic outcome. Strahlenther Onkol. 2018. DOI: 10.1007/s00066-018-1346-7
- Cardoso JS, Cardoso MJ. Towards an intelligent medical system for the aesthetic evaluation of breast cancer conservative treatment. Art Intel Med. 2007;40:115–126.
- Verhoeven K, Kindts I, Laenen A, et al. A comparison of three different radiotherapy boost techniques after breast conserving therapy for breast cancer. Breast. 2015;24:391–396.
- Offersen BV, Boersma LJ, Kirkove C, et al. ESTRO consensus guidelines on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114:3–10.
- Bentzen SM, Agrawal RK, Aird EG, START Trialists’Group, et al. The UK standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9:331–341.
- Hanley J, McNeil B. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36.
- Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–138.
- Mukesh M, Harris E, Jena R, et al. Relationship between irradiated breast volume and late normal tissue complications: a systematic review. Radiother Oncol. 2012;104:1–10.
- Immink JM, Putter H, Bartelink H, et al. Long-term cosmetic changes after breast-conserving treatment of patients with stage I-II breast cancer and included in the EORTC 'boost versus no boost' trial. Ann Oncol. 2012;23:2591–2598.
- Poortmans P, Bartelink H, Horiot JC, et al. The influence of the boost technique on local control in breast conserving treatment in the EORTC 'boost versus no boost' randomised trial. Radiother Oncol. 2004;72:25–33.
- Shiina N, Sakakibara M, Fujisaki K, et al. Volumetric breast density is essential for predicting cosmetic outcome at the late stage after breast-conserving surgery. Eur J Surg Oncol. 2016;42:481–488.
- Rosenstein B, West C, Bentzen S, et al. Radiogenomics: radiobiology enters the era of big data and team science. Int J Radiat Oncol Biol Phys. 2014;89:709–713.
- Forrester H, Li J, Leong T, et al. Identification of a radiation sensitivity gene expression profile in primary fibroblasts derived from patients who developed radiotherapy-induced fibrosis. Radiother Oncol. 2014;111:186–193.
- Quarmby S, West C, Magee B, et al. Differential expression of cytokine genes in fibroblasts derived from skin biopsies of patients who developed minimal or severe normal tissue damage after radiotherapy. Radiat Res. 2002;157:243–248.
- Lyngholm C, Overgaard J, Christiansen P, et al. Validation of a gene expression profile predictive of the risk of radiation-induced fibrosis in women treated with breast conserving therapy. Acta Oncol. 2015;54:1665–1668.
- Burnet N, Elliott R, Dunning A, et al. Radiosensitivity, radiogenomics and RAPPER. Clin Oncol (R Coll Radiol). 2006;18:525–528.
- Barnett G, West C, Dunning A, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer. 2009;9:134–142.