 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background: The incidence of surgery due to metastatic bone disease in the extremities (MBDex) and postoperative survival remain uninvestigated in the population. The aim of the current study was: to identify (1) incidence, demographics and survival of a population-based cohort of patients having surgery for MBDex (2) rate of referrals and referral pattern to a musculoskeletal tumour centre (MTC).
Material and method: A prospective study of a consecutive population-based cohort of patients having surgery for MBDex from 2014 to 2016. Patient demographics, indication for surgery, oncological status, and postoperative survival was obtained from patient interviews, surveillance scans and patient records.
Results: We identified 164 patients treated for 175 bone lesions resulting in an incidence of MBDex surgery of 48.6 lesions/million inhabitants/year and a 10% risk of undergoing surgery for MBDex for every year liven with metastatic bone disease. The most common primary cancers were breast, lung, renal, prostate and myeloma. Twenty-nine lesions represented debut of cancer and 22 lesions debut of relapse of a previous cancer. Overall one-year survival was 41% (95% C.I.: 33%–48%). Fifty-nine percent of patients were referred for treatment at MTC. Patients referred had better prognostic baseline characteristic than patients treated at secondary surgical centres (SSC) (lower ASA score (p < .001), no visceral metastasis (p < .001), lower age (p < .001) and less aggressive primary cancer (p < .001)). The one-year probability of overall survival was higher for MTC patients compared to SSC patients (p < .001).
Conclusions: Present study describes a prospective population-based cohort of patients having surgery for MBDex identifying incidence and postoperative survival. Referral of patient is biased by selection where ‘long-term survivors’ are referred for treatment at MTC. We can, however, not exclude that treatment centre influences chance of survival after surgery for MBDex although our study was not designed to identify any potential influence.
Introduction
Dissemination of cancer to the skeleton is common for patients diagnosed with cancer with reported rates from 30% [Citation1] to 50% [Citation2] in autopsy studies which probably reflect the multiple primary cancers and their different potential to disseminate to the bone. Likewise, literature reports very diverse patient survivals after surgery for metastatic bone disease in the extremities (MBDex). Kirkinis et al. [Citation3] found in a review, one-year overall survival rates ranging from 17% to 69.5% after surgery for MBDex. Different mechanisms of confounding are inherent to the published literature and could in part explain this variation: reports from selected populations only reflect the outcome of selected patients treated at specialised musculoskeletal tumour centres (MTC) (survival biased by indication for referral); different institutional policies on indication for surgery in impending lesions may cause time bias of reported survival, because patients will live longer if treated primarily for an impending fracture, instead of waiting until fracture occurs (introducing lead time bias). Finally, survival estimates from patients operated prior to the introduction of targeted oncologic treatment might not capture the expected gain in survival from this.
Hitherto no population-based study of surgery due to MBDex has been performed. Therefore, the incidence of MBDex related surgical interventions, postoperative survival and demographic composition of this patient group remains unknown.
The aim of this study was to investigate the incidence of surgical interventions for MBDex, the postoperative patient survival, and the epidemiological composition (demographics) in a population-based prospective cohort. Secondly, we aimed at investigating the referral rate for treatment at MTC and a possible selection bias in referral patterns between patients treated at MTC and secondary specialised centres (SSC).
Material and methods
We conducted a prospective observational study of all patients living in the Capital Region of Denmark (CRD) and having surgery due to MBDex in a two-year period from 19 May 2014 to 18 May 2016. The number of inhabitants living in the CRD is 1.8 million people (31% of the Danish population), which is considered a reliable cross-section of the entire population [Citation4]).
Ethical approval was obtained from the regional ethical committee (ID.nb.: H-4-2014-005) and the Danish Data Protection agency (ID.nb.: 30-1222).
Patients were identified if preoperative imaging showed signs of malignant disease in the bone or if the malignant disease was suspected by the history of fracture mechanism. Every treatment centre in the CRD had an appointed study investigator to identify the patients and report back to primary investigator (MS). Furthermore, to ensure complete inclusion, the preoperative images from all patients having orthopaedic surgery in the CRD were systematically screened by one investigator (MS) in the inclusion period.
The five CRD hospitals are licensed to treat orthopaedic patients, and no patients suffering from MBDex can be expected to be treated outside these centres due to the social healthcare system in Denmark. If a patient experienced an acute fracture during e.g., a holiday outside the CRD and hereby needed surgery outside the CRD, the patient would always be transferred back to a regional hospital for rehabilitation. Hence, all patients from CRD having surgery due to MBDex can be expected to be included in this study, thus providing a true population-based cohort.
Referral to MTC was purely based upon decisions by the local orthopaedic department and sometimes solely on the local on-call surgeon. The present study had no influence on the treatment strategy. In general, patients are referred for treatment at MTC in our region in cases of advanced bone loss and when the need for bone resection and reconstruction is considered necessary by SSC surgeons.
Patients were excluded from the study if histopathology showed no signs of malignant disease. If histopathology was not ensured during surgery, or if the biopsy material was not suitable for histopathological analysis, the patient/lesion was followed for one year. If no progression of the lesion was observed, the lesion was considered non-malignant. If the patient died in close relation to surgery and therefore no progression of a lesion could be expected, the case was evaluated by a multidisciplinary team including a senior consultant musculoskeletal tumour surgeon and a musculoskeletal radiologist, and they decided if the lesion was highly likeable to be malignant or not.
All patients were included prior to surgery or, in case of acute surgical intervention, in immediate coherence to surgery.
Data regarding the prevalence of metastatic bone disease in the Danish population was obtained from the Danish National Patient Registry (DNPR) [Citation5] and was used to calculate the risk of undergoing surgery for MBDex for patients diagnosed and living with metastatic bone disease. DNPR was founded in 1976 and include all data (ICD-coding) from every hospital admissions (since 1977) and outpatient clinic visits (since 1995). Previously the ICD-10 coding DC 79.5 (condition with bone metastasis) has been validated in the DNPR by Jensen et al. [Citation6] with a positive predictive value of 92.6%.
Preoperative variables
Karnofsky performance status score (KPS) [Citation7] was estimated retrospectively one month prior to surgery in case of acute fracture by patient interview at inclusion.
From the Danish National Pathology Registry [Citation8], we identified histopathological diagnosis and date for debut of primary cancer causing the lesion. If primary cancer was unknown and no previous cancer was diagnosed, the date of diagnosis was considered the same as the date of surgery. If primary cancer was diagnosed outside Denmark and the precise date was unknown to the patient, it was set to the first calendar day of the month of diagnosis.
From patient interviews and the oncologist records, the following variables were obtained: presence of visceral metastases (in case of no prior screening for disseminated disease, imaging performed up to three months after surgery was used to estimate this variable at baseline), number of bone metastases (same approach as visceral metastases), age, gender, anatomical site of lesion, and it was recorded in the treated lesion represented the debut of cancer or relapse of a previous primary cancer.
Perioperative variables
From the surgical notes and implant lists, we identified surgical technique (bone resection or stabilisation), implants used (devices for internal fixation, prostheses), and if representative histopathology material was obtained.
Follow-up
Patients were followed until the end of study (18 May 2017) or death. No patients were lost to follow-up due to the Danish Civil Registration System that ensures accurate account for emigration and/or death [Citation9].
Missing data
Residual disease (bone metastases and visceral metastases): whole body scans were evaluated if they were performed within the last three months before or three months after surgery. If no scans were performed during this period, the variable was considered missing.
ASA score: Was considered missing if the anaesthesiologist did not include this information in the preoperative evaluation prior to the surgery.
Statistical analysis
All statistical calculations were performed using R (R Foundation, Vienna, Austria) [Citation10]. Confidence intervals (C.I.) were considered as 95% of normal distribution and the statistical significance level was set at p < .05.
Subgroups were analysed by treatment centre (MTC versus SSC) and tested for statistical significance using Mann–Whitney-Wilcoxon test for continuous variables and Chi2 test for categorical variables.
Patient survival was addressed using Kaplan–Meier estimate for cumulated survival and the difference between survivals in patients related to treatment centre was evaluated by log-rank test. Censoring was always right censoring and patients was included into survival analysis only at index surgery.
Results
Incidence of surgery, demographics and survival
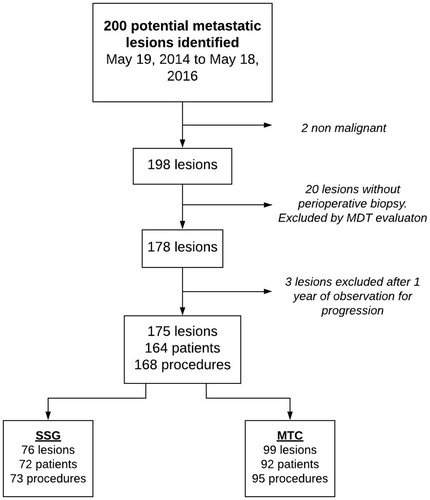
Two-hundred lesions were suspected of being malignant by initial screening of X-rays and patient history. Two lesions were without findings of malignant disease in histopathological specimens. Twenty-three lesions had no per-operative biopsy performed. After evaluation of history, X-rays, CT and/or MRI scans in a multidisciplinary setting, 20 lesions were considered not malignant and thus excluded. Three lesions were suspected to be malignant after multidisciplinary evaluation but showed no signs of progression of the suspected lesion after one year, and thus MBDex was ruled out (all three received internal fixation with no removal of the lesion or treatment with local/systemic therapy). Thus, we identified 164 patients with 175 MBDex lesions () that were treated with 168 surgical procedures during the two-year period. This results in an incidence of 48.6 surgically treated MBDex lesions per million inhabitants in the CRD per year.
Figure 1. Flow diagram illustrating identification of the cohort of patients treated operatively for metastatic bone disease of the extremities in the Capital Region of Denmark (total population 1.81 million) from 19 May 2014 to 18 May 2016. MDT: highly specialised multidisciplinary team; MTC: musculoskeletal tumor center; SSG: secondary surgical centers.
During the inclusion period of two years in the present study, 5,335 person-years were accumulated for patients living with bone metastases in the Danish population (ICD-10 code DC79.5). Currently, the Danish population counts 5.76 million people with 1.81 million living in the CRD [Citation4]. With 164 patients undergoing surgery for MBDex in the CRD we found that
of patients living one year with bone metastases in the CRD will need surgical treatment for an extremity lesion.
Cancer of the breast, lung, kidney, and prostate plus myeloma were the most common types of primary cancer-causing MBDex. Twenty-nine lesions (17%) represented the debut of cancer and 22 lesions (13%) the debut of a cancer relapse (). In 14 patients, no regular surveillance scans had been performed, and eight patients that had regular scans performed the lesions were not identified due to insufficient scanning with the scan area not including the particular part of the extremities. Of the 29 lesions that represented the debut of a cancer disease, sufficient biopsy material was obtained in 19 of the cases. In three cases the biopsy was insufficient, and in the remaining seven cases no biopsy was obtained. In these cases, the suspicion of a metastatic lesion was withheld based on imagining, and primary tumour was identified by PET scans. In three lesions, no biopsies were performed, and the primary tumours were never identified: all three patients were treated at SSC.
Table 1. Table describing and comparing the preoperative data of the cohort of patients treated operatively for metastatic bone disease of the extremities in the Capital Region of Denmark (total population 1.81 million) from 19 May 2014 to 18 May 2016.
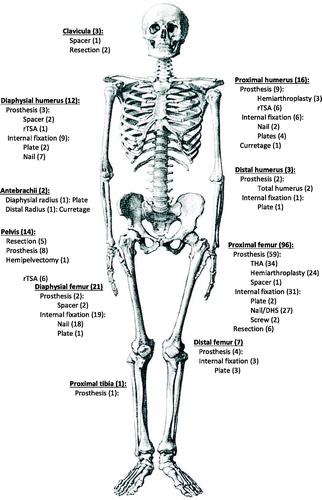
One-hundred and forty-one lesions (81%) were located in the lower extremities/pelvis and 34 lesions (19%) were located in the upper extremities. The most frequent anatomic location of the MBDex lesions was the proximal femur. Ninety-six lesions (55%) were treated with a prosthesis, 10 lesions (6%) were resected without reconstruction and 69 lesions (39%) were stabilised with an internal fixation device ().
Figure 2. Figure illustrating the anatomical sites of treated metastatic lesions and method of surgical management of the cohort of patients treated operatively for metastatic bone disease of the extremities in the Capital Region of Denmark (total population 1.81 million) from 19 May 2014 to 18 May 2016. rTSA: reverse total shoulder arthroplasty; THA: Total Hip Arthroplasty.
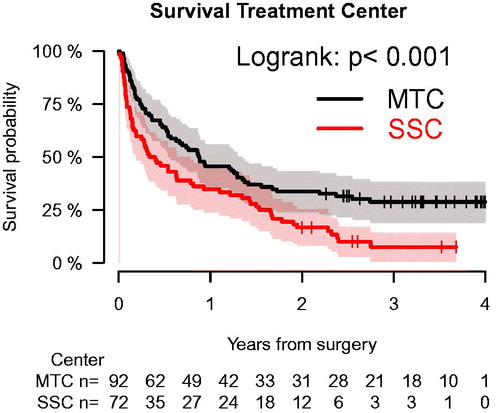
The estimated cumulated probability of overall survival for this population-based cohort was 41% (95% C.I.: 33%–48%) at one year, 27% (95% C.I.: 20%–34%) at two years and 21% (95% C.I.: 13%–28%) at three years.
The mean time to death for the entire cohort was 211 days (range: 0–930), 225 days (range: 4–930) for MTC, and 197 days (range: 0–871) for SSC patients. We found a statistically significant difference in survival between patients having surgery at MTC versus SSC (p < .001) ().
Figure 3. Kaplan–Meier survival analysis showing cumulated overall survival for a population-based cohort of patients having surgery for metastatic bone disease in the extremities in the Capital Region of Denmark (total population 1.81 million) stratified for treatment center. Patients were only included into analysis at index surgery during the study (n = 164). Overall one-year survival for the entire cohort was 41% (95% C.I.: 33%–48%) and 46% (95% C.I.:36%–56%) versus 35% (95% C.I.: 24%–46%) for patients treated at a musculoskeletal tumor center or a secondary surgical center, respectively (p < .001).
Patient referral and referral pattern
Fifty-nine percent of patients undergoing treatment for MBDex was referred for highly specialised treatment at MTC from SSC. Patients treated at MTC had a statistically significant better preoperative status with regard to known prognostic variables compared to patients treated at SSC. We found that a patient referred to a MTC were characterised by lower ASA score (p < .001), younger age (p < .001), a less aggressive cancer disease (p < .001), impending fracture (p < .001), no visceral metastases or a longer interval from diagnosis of primary disease to surgery for the metastatic lesion (p < .001) ().
There was no difference between SSC and MTC related to the presence of multiple bone metastases, spinal metastases, if the lesion was the debut of cancer or relapse, or preoperatively irradiated. Mean KPS for MTC patients (mean 76, range: 20–100) versus SSC patients (mean 73, range: 20–100) showed no statistically significant difference in distribution (p = .324) ().
Discussion
A great variety in postoperative survival after surgery for MBDex has been reported in the literature, and this is probably a reflection of selection bias, but could also reflect improvement in oncologic treatment throughout time, although a recent study was not able to identify such a gain in survival after surgery for MBDex [Citation11]. We need a better understanding of how a population of MBDex patients is composed in order to recognise and stratify patients to surgery.
We found an incidence of 48.6 MBDex lesions surgically treated/million inhabitants/year. To our knowledge only Tsuda et al. [Citation12] has performed a similar study in 2016, however, it was based on database coding focusing only on femur metastatic lesions. They found an incidence of only 2.9 metastatic femur lesions/million inhabitants/year compared to 35 femur lesions/million inhabitants/year in our study.
Comparison of our study with the Japanese study [Citation12] underlines the importance of caution in interpretation of studies conducted on databases without national coverage as one cannot expect them to represent the entire population due to selection bias. In the present study, we also found a selection bias regarding patients with good performance status to be referred to a highly specialised centre and a concomitant better survival in this part of our cohort compared to patients treated at SSC. These findings are important to bear in mind in the interpretation of studies from selected cohorts of patients being treated only at highly specialised centres.
The prevalence/incidence of MBDex in a population is difficult to quantify partly due to the sensitivity of the diagnostic tools used [Citation13] and the extent of surveillance scans performed after the primary diagnosis. The authors feel that the findings of the present study contribute to a better understanding of the number of patients suffering from these complications to a cancer diagnosis. In our opinion, it is important to match the expectations of the patients, and therefore we calculated the actual risk of undergoing surgery for MBDex in patients living with bone metastases and found it to be 10.4%.
In the present study, 13% of the lesions represented relapse of a previous cancer and in 17% the debut of cancer. This is coherent with findings in the database study from the Scandinavian Sarcoma Group Skeletal Metastasis Registry that report 14% of treated lesions represented the debut of cancer and 12% represented relapse of a previous cancer, even though the distribution of cancer types were not identical to our study. As a substantial proportion of relapse patients in the present cohort are treated at a SSC without obtaining proper biopsy material, our study underlines the conclusion of the study by Cummings et al. [Citation14]; even after decades of guidelines for surgical treatment for MBD, there remains a low awareness in SSC for the treatment principle/options since these patients should have been referred for highly specialised multidisciplinary team evaluation and probably a preoperative biopsy to rule out primary/new malignancy. A missing biopsy in these cases can cause a delay in diagnosis and treatment and even potentially influence the survival of the patients.
Because of the prospective design and population-based settings, this study has few limitations. However, since this is a cohort representing a Scandinavian population, where treatment of MBDex is anchored in government-financed hospitals. Thus, the treatment strategy in the present study might not reflect other populations in countries with different healthcare systems or policies on the indication for operative treatment of MBDex. The strength of the present study is that no patients were lost to inclusion. The authors emphasise that every surgery was based on a case by case decision from attending physician, and no interference from study investigators was present, hereby minimalizing confounding.
Since the present study is observational, selection bias of patients with good prognostic factors for survival of treatment at MTC is present. We attribute the increased survival for patients treated at MTC to this selection bias, however, we cannot, based on the current study design, exclude that treatment centre influenced the probability of survival.
The calculation of the risk of undergoing surgery for MBDex is based on ICD-10 coding for bone metastases, it might be underestimated, as the code does not differentiate between patients having only spinal metastases (and therefore not in risk of having surgery for MBDex) and patients having metastases in the extremities. As no code to differentiate these two patient categories exists, calculating as described here was the only way of means.
Lastly, as literature describes, there is a great variance of individual patient survival and caution should be made in generalising this percentage to the individual patient. Several models for prediction of survival after surgery for MBDex has been published and are better suited for this purpose [Citation15–17]. However, this study provides, for the first time, a cohort that can validate these systems and contribute to investigate how the models perform in an unselected patient cohort and hereby aid to test their calibration and robustness.
In conclusion, the estimated survival after surgery for MBDex can be expected to be ∼41% one year after surgery in a general unselected patient population with a 10% risk of undergoing surgery for MBDex in case of dissemination of cancer to the skeleton. Higher survival was found in patients referred for specialised treatment at MTC and can be explained by selection bias by referral pattern.
The incidence of metastatic lesions in need of surgical intervention is NOT low and should be kept in mind when treating atypical fractures or spontaneous fractures of the limbs. Orthopaedic surgeons should address and further investigate a suspected malignant lesion prior to treatment by obtaining proper histopathological material to diagnose debut of cancer aiming for eliminating whoops procedures and ensuring correct diagnosis without delay. Oncologist could benefit from including the subtrochanteric area in their planned surveillance scans performed as follow-up after primary cancer treatment have been completed bearing in mind that 47% of lesions (60 lesions out of 127 lesions) in the current study were not identified on regular surveillance scans as they did not include the subtrochanteric area of the femur.
Acknowledgments
We thank all orthopaedic surgeons and personnel in the CRD who have contributed to this study by reporting to authors when a suspected metastatic lesion was operated. Also, a great thank to Karl Erik Jensen MD, DMSc, Department of Diagnostic Radiology, Rigshospitalet, Copenhagen University Hospital for further evaluating diagnostic imaging if in doubt of malignant lesion.
Finally, a great thank to all the staff at Musculoskeletal Tumour Section, Department of Orthopaedics, Rigshospitalet, Copenhagen University Hospital for support to this study – it has been more than one could expect!
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3:74–85.
- Buckwalter JA, Brandser EA. Metastatic disease of the skeleton. Am Fam Physician. 1997;55:1761–1768.
- Kirkinis MN, Lyne CJ, Wilson MD, et al. Metastatic bone disease: a review of survival, prognostic factors and outcomes following surgical treatment of the appendicular skeleton. Eur J Surg Oncol. 2016;42:1787–1797.
- Danmarks statestik. 2017 [12.10.2017]. Available from: http://www.statistikbanken.dk/statbank5a/default.asp?w=1680.
- Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39:30–33.
- Jensen AO, Norgaard M, Yong M, et al. Validity of the recorded International Classification of Diseases, 10th edition diagnoses codes of bone metastases and skeletal-related events in breast and prostate cancer patients in the Danish National Registry of Patients. Clin Epidemiol. 2009;91:101–108.
- Karnofsky DA, Abelmann WH, Craver LF, et al. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–656.
- Bjerregaard B, Larsen OB. The Danish Pathology Register. Scand J Public Health. 2011;39:72–74.
- Schmidt M, Pedersen L, Sorensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549.
- R Core Team. (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
- Hovgaard TB, Horstmann PF, Petersen MM, et al. Patient survival following joint replacement due to metastatic bone disease - comparison of overall patient and prostheses survival between cohorts treated in two different time-periods. Acta Oncol. 2018;2:1–10.
- Tsuda Y, Yasunaga H, Horiguchi H, et al. Complications and postoperative mortality rate after surgery for pathological femur fracture related to bone metastasis: analysis of a nationwide database. Ann Surg Oncol. 2016;23:801–810.
- Lange MB, Nielsen ML, Andersen JD, et al. Diagnostic accuracy of imaging methods for the diagnosis of skeletal malignancies: a retrospective analysis against a pathology-proven reference. Eur J Radiol. 2016;85:61–67.
- Cumming D, Cumming J, Vince A, et al. Metastatic bone disease: the requirement for improvement in a multidisciplinary approach. Int Orthop. 2009;33:493–496.
- Sorensen MS, Gerds TA, Hindso K, et al. Prediction of survival after surgery due to skeletal metastases in the extremities. Bone Joint J. 2016;98-B:271–277.
- Forsberg JA, Eberhardt J, Boland PJ, et al. Estimating survival in patients with operable skeletal metastases: an application of a bayesian belief network. PLoS One. 2011;6:e19956.
- Janssen SJ, van der Heijden AS, van Dijke M, et al. 2015 Marshall Urist Young Investigator Award: prognostication in patients with long bone metastases: does a boosting algorithm improve survival estimates?. Clin Orthop Relat Res. 2015;473:3112–3121.
