Abstract
Background: Knowledge of chondrosarcoma (CS) of bone to date is based on institutional reports and registry publications with limits in reporting, detail and quality of data.
Method: We have performed a retrospective search of CS of bone in the National Cancer Registry in Norway from 1990–2013, cross checked against local tumor databases with further quality control and supplementation of all data from clinical files. The time period is defined by the routine use of axial imaging in clinical practice. A total of 311 cases are included. We performed 108 pathological reviews and 223 radiological reviews. The manuscript was prepared according to the STROBE checklist for strengthening of observational studies. We performed uni-/multivariate cox analyses to define independent prognostic variables from the main cohort of central CS of bone.
Results: The incidence of CS of bone in Norway is 2.85/million/yr. for both sexes overall, rising to 3.45/million/yr. in the last 5-year period. There is an increase in the most common central CS subtype, stronger for women than for men.
Central CS had, in general 10–15% local recurrence rates, all evident by 5 years while metastasis rate increases with location and grade. Exceptions are extremity grade 1 CS which displayed no metastatic events and axial grade-3 disease with high rates (50%) of both local and metastatic relapse. Peripheral CS had limited metastatic potential (2%), but rates of local relapse (13%) continue to appear towards 10 years of follow up.
Malignancy grade 3 independently predicts rate of metastasis and presence of soft tissue component predicts local recurrence, metastasis and survival.
Conclusion: Rates of local recurrence, metastasis and disease specific survival follow clear patterns depending on subtype, location and grade allowing better tailoring of follow-up regimes. Malignancy grade 3 and the presence of a soft tissue component independently predict behavior for central CS of bone.
Introduction
Background
Numerous studies regarding chondrosarcoma (CS) of bone start by saying that ‘chondrosarcoma is the second most common primary malignant tumor of bone [Citation1–3].’ Knowledge concerning the epidemiology of CS is however somewhat unreliable as it is largely based on institutional reports [Citation1–5].
There are also registry publications on CS epidemiology, from the Surveillance, Epidemiology and Results (SEER) database in the USA [Citation6,Citation7], the National Cancer Intelligence Network (NCIN) in England [Citation8] and the International Agency for Research on Cancer (IARC) [Citation9]. The SEER database is estimated to represent up to 30% of the national U.S. population while the NCIN have converged reporting from eight regional cancer registries in the United Kingdom. In neither has there been review, quality control of data included or confirmation of the histopathological diagnosis. Uniformly these registries have limited variables and chondrosarcoma is analyzed as one disease rather than at subtype level.
Valery et al report from the IARC on overall primary bone cancer incidence by morphological subtype [Citation9]. The proportion of CS varied substantially, ranging from <10% in India and Saudi Arabia to over 45% in Finland, Slovenia and the Netherlands. Indeed, in six countries, CS is reported to be the most common primary malignant bone tumor. These are all countries with high levels of morphologically verified inclusion. Anfinsen et al [Citation6] found a 50% increase in incidence of CS for females aged 20–69 years from the mid 1980s onwards from the SEER database. Whelan et al [Citation8] also report an increase in incidence of CS for both sexes in the United Kingdom in the 1980s with a subsequent stabilization.
The study of prognostic factors for CS of bone are also limited. Like epidemiology studies, they often cover long observation periods [Citation3,Citation5], report on all chondrosarcoma as a single disease [Citation10,Citation11], focus on a specific anatomical location [Citation12] or have limited multivariate analysis [Citation1,Citation2] or review.
Large scientific breakthroughs in CS research are lacking, but there has been a gradual inclusion of a number of important lesser developments over the last few decades. These include the differing etiology and biology of peripheral and central subtypes; challenges in interpreting biopsy specimens [Citation13]; widespread heterogeneity within chondroid tumors; safe use of curettage for grade 1 extremity intramedullary disease [Citation14–21] as well as the role of modern axial imaging to depict tumor biology [Citation22–29] among others. The recognition and inclusion of these factors in defining a valid cohort, study period and detail level are vital to support meaningful conclusions transferable to modern clinical practice.
Concurrently, there is increasing focus on the low level of evidence used in decision making regarding primary bone tumors [Citation30]. This challenge is being met by consensus guidelines for the strengthening of reporting of observational studies [Citation31].
The aims of the present study are first to define the incidence of CS of bone in Norway in a modern era and describe rates of recurrence, metastases and survival at a subtype level. Secondly, to present multivariate prognostic analysis of factors influencing local recurrence, metastasis and survival for the main cohort of central CS.
Methods
Recruitment
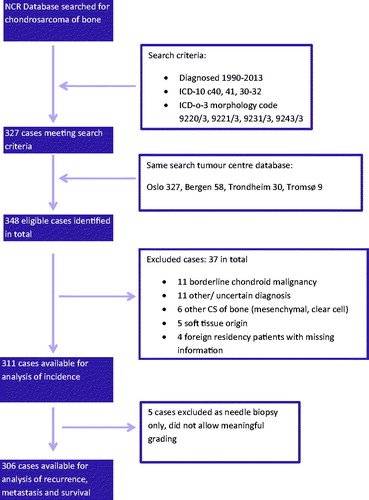
We searched the National Cancer Registry (NCR) for patients from 01 January 1990 till 31 December 2013 (). The time period is chosen based on the routine use of CT/MRI imaging in clinical practice. We have searched for diagnostic ICD-10 codes C40, 41 and 30-32 while correlating these with ICD-0-3 morphology codes 9220/3, 9221/3, 9231/3 and 9243/3. The initial NCR search produced 327 cases. The same search was performed at the tumor database of the four referral centers for bone pathology in Norway. A number of patients were identified at more than one center. In total we found 348 eligible cases. The NCR had 38 (11%) cases not located in the hospital databases. We found only three patients at the tumor centers not registered at the NCR, though 18 cases were registered with wrong topography or morphology codes.
We retrieved all data related to primary disease status, treatment and follow-up. This was then reviewed and complemented by the main author based on predetermined definitions from the clinical files for all cases ensuring quality control of all data.
Review
Radiological review was performed on all 223 cases with available imaging.
Histopathological review was done on microscopy slides taken from operative specimens. This includes both resection specimens and curettage specimens. 5 cases with only a needle biopsy were included in the calculation of total incidence without grade, but then removed from further analysis. Twenty cases whom did not undergo definitive sarcoma surgery have open biopsies or samples from unplanned/contaminated surgeries.
Histopathological review was performed if there were any uncertainty or contradictions in the text of the pathology report or clinical files relating to unclear pathological grade (46 cases); unspecified or uncertain type of CS (18 cases); unusual biology (8 cases), missing information (20 cases); or other doubt regarding diagnosis (20 cases). This resulted in 108 pathological reviews. 68 were performed by Dr Bjerkehagen; senior sarcoma pathologist in Oslo. The remaining 40 have been assessed at a meeting of the Norwegian mesenchymal tumor board with pathologists from all 4 regional university tumor centers.
This resulted in the exclusion of 37 cases; 11 with borderline malignant chondroid diagnosis; 11 with other/uncertain diagnosis; 6 with other CS (mesenchymal, clear cell); 5 with soft tissue origin and 4 patients with foreign residency and thereby missing information. The final cohort consists of 311 cases of morphology verified CS of bone of central, peripheral, periosteal and dedifferentiated subtypes available for analysis. For analysis of outcome, a further five patients were excluded due to their having only needle biopsy performed and thereby no reliable malignancy grade.
Definitions
Grading has been practised in accordance with Evans [Citation32] and WHO criteria in a four-grade system, with increasing weighting of radiological signs of aggressiveness through the 1990’s. The term ‘low grade’ is meant to define grades 1 and 2, while ‘high grade’ denotes 3 and 4. This has been the practice in Norway during the study period and is similar to comparable articles [Citation7]. Central, peripheral and periosteal subtypes are graded from 1–3 while dedifferentiated CS of both central and peripheral subtypes have by definition been defined as grade 4 [Citation7,Citation33].
Head & neck lesions include nasal, laryngeal, facial bones and skull base tumors.
Chest wall cases arising from the costochondral cartilage have been grouped with central subtypes.
Anatomic definitions of the axial/appendicular skeleton define scapular and pelvic CS as appendicular location. In CS literature this definition varies. Since the biology and treatment of scapular and pelvic lesions are similar to other axial locations we have chosen to use the glenohumeral and hip joints to discriminate lesions into axial or extremity location.
We have included one patient with a ‘high grade central CS’ and 10 with ‘low grade’ where specimens were not available for review. These are all from the early part of the study period. The case of high grade is not dedifferentiated and as such denoted grade 3. The 10 low grade cases have all been grouped as grade 2 cases since it is common practice to let the area of highest grading define the grade and more importantly all 10 are central CS cases with proven soft tissue components.
The dedifferentiated cohort consists of 35 central subtypes and 4 peripheral subtypes. If not otherwise stated they are analyzed together.
We have used the NCR definition ‘dead from cancer’ as depicting disease-specific survival (DSS) with censor date 30 October 2016; linked to the national death registry.
The surgical margin has been translated and scrutinized by the main author from the Enneking system to the UICC Residual tumor system based on pathology reports and clinical notes. A successful curettage has been denoted with R1 status.
‘Unplanned surgery’ is meant to convey contaminated surgery. That is surgery performed without the intent of being curative for known/presumed chondrosarcoma.
Follow-up
Centralization of bone tumor services in Norway is longstanding and follow-up is organized by standards set by Scandinavian Sarcoma Group (SSG) and European Society for Medical Oncology (ESMO). Follow-up for CS of bone is for minimum 10 years and entails clinical and radiological examination of diseased location as well as radiological assessment of metastatic stations. Adherence to this policy is strict with only a few exceptions for elderly patients with cumbersome travel arrangements. In such cases, the follow-up is organized locally.
Ethics
All retrieval and storage of data has been approved by relevant regional and institutional authorities. The project is approved and based at the NCR. Data retrieval is founded on the quality control charter of the cancer registry act of 1967, last updated in 2014. The Regional Ethics Board (REK) of the south of Norway health area has been consulted and accepted this foundation.
Statistics
Stata 14 software was used for statistical analysis. We present descriptive statistics of the cohort and Kaplan-Meier estimates for rates of local recurrence (LR), rates of metastasis (Met) and disease specific survival (DSS) at 2, 5 and 10 years of follow-up, as well as Kaplan–Meier curves. Log-rank test was used to test differences in survival curves. Statistical significance has been set at p < .05. Incidence has been calculated based on annual population data provided by Statistics Norway (SSB) in Excel with 95% confidence intervals calculated assuming a Poisson distribution.
We present uni- and multivariate analyses by cox proportional hazard models for a cohort of central CS of bone excluding head and neck locations (no. 197). Models were constructed to include previously published variables of importance and those used in modern clinical practice to depict CS behavior. All models passed the test of proportional hazards. We report categorical variables except for age which was analyzed as a continuous variable. Multivariate models predicting local recurrence or metastasis include age at diagnosis, sex, extremity/axial location, tumor size according to AJCC standards (>8 cm), presence of a soft-tissue component and malignancy grade. The model for DSS also included metastasis at diagnosis. We report hazard ratios with 95% confidence intervals and likelihood-ratio (lr) tests.
Bias
We have made several attempts at correcting for selection bias. Primarily, we have used multiple sources for data recruitment and performed review of as many cases as possible. There is a possibility for systematic bias since the main author has performed all data collection and quality control. This has, however, been according to predetermined definitions and both missing information and discrepancies have been defined by patho-radiological review, partly in a group setting.
The manuscript has been prepared in accordance with the ‘STROBE checklist’ for observational studies as far as the methodology allows [Citation31].
Results
Incidence
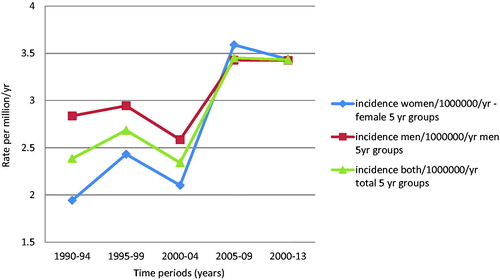
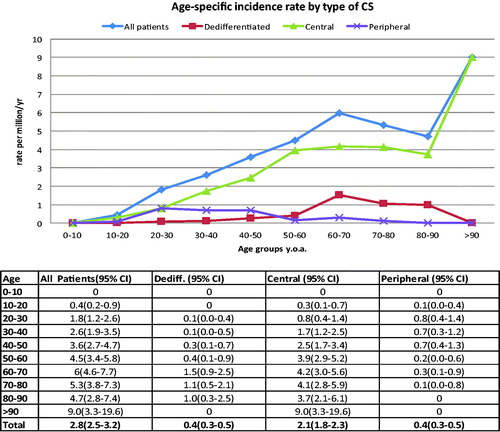
The average overall incidence of CS for the study period was 2.85 per million per year (2.67 for women, 3.04 for men). There was a definite trend towards increasing incidence for both sexes during the study period (). This was more pronounced for women so that the incidence appears equal among the sexes and overall at about 3.45 per million from 2005–2013. There was also a clear increase in incidence with age for both sexes and overall (). The incidence curve for all types appears to be driven by the numbers for central CS. This was both the most common subtype and had a steady increase in the study period and with age. The sharp increase in the age group over 90 years of age (y.o.a.) is likely due to the fact that the total population in this age group is markedly smaller and should be interpreted with caution. Dividing central types into grade and looking at the first and last 10 years of the study period; all grades increased, but most of all grade 2 lesions ().
Table 1. Incidence over time by grade, all types.
Cohort
depicts the patient demographics of the studied cohort. It shows an overall equal gender split with mean age 55 y.o.a. The exception to this was peripheral subtype with a male:female ratio of 1.8:1 and mean age of 35 y.o.a. Both peripheral and dedifferentiated subtypes have on average larger tumors than central CS and overall. While central CS appeared equally as often in the extremities as in axial skeleton, peripheral and dedifferentiated subtypes appeared approx. 90% in the extremities and only 10% in the axial skeleton. Peripheral CS presented mostly as grade 1 malignancy grade (56%) while central CS presented more evenly as 33% grade 1, 40% grade 2 and 24% grade 3 disease, respectively. Extremity central CS presented as 46% grade 1, 34% grade 2 and 20% grade 3 while axial central CS as 21%, 49% and 30%, respectively. While 41% of the peripheral CS population had an underlying syndrome (multiple osteochondromatosis), this was much more seldom (approx. 5%) for central CS (Ollier/Maffucci) and dedifferentiated types. Grade 1 CS was most common in early adulthood with a gradual decline with age. Dedifferentiated disease conversely, presented first in the third decade of life and gradually increased in occurrence.
Table 2. Patient cohort.
Events
Rates of LR, Met and DSSare shown in .
Table 3. Rates of local recurrence, metastasis and disease-specific survival.
Local recurrence rates were similar for most types and grades of chondrosarcoma at about 10-15%. Most LR were evident within 2 years and all by 5 years. Peripheral CS is the exception with LR appearing also after 5 years. Notably, axial grade 3 central CS and dedifferentiated CS had higher rates of recurrence at approx. 50%, but again with a clear trend towards early events and stable rates from 5 years of follow-up and onwards.
Metastatic events occurred overall at a rate of 15% at 5 years. Most metastases appeared before 2 years and some further up until 5 years but with stable patterns from 5–10 years of follow-up. For central CS rates of metastasis were higher for axial disease than extremity (p < .001) and with a clear increase according to malignancy grade. Metastasis in peripheral CS was rare (2%) and none after 2 years of follow-up. Dedifferentiated CS conversely had a high metastasis rate of 65%, but also stable after 2 years follow-up. Periosteal and central grade 1 extremity CS had no metastatic events in this study.
Survival
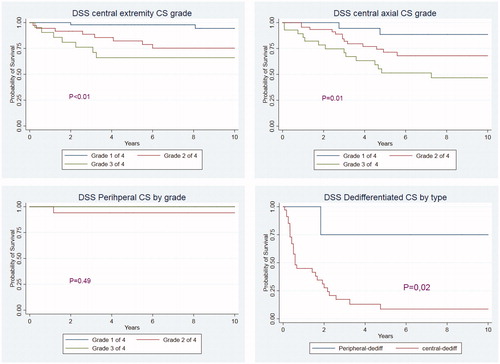
Overall survival was about 5% lower than DSS. Survival was strongly dependent on malignancy grade (p < .001) and patients with central CS of extremities fared better than central axial CS overall (p = .01). DSS is also strongly dependent on subtype of chondrosarcoma (p < .001). Splitting dedifferentiated CS survival curves in to central and peripheral disease we see that peripheral dedifferentiated chondrosarcoma has DSS similar to central chondrosarcoma overall with 2, 5, 10 year DSS rates of 75%, 75%, 75% while central dedifferentiated disease has much more serious outcome with 2, 5, 10 year DSS of 28%, 9% and 9%. depicts Kaplan–Meier curves for central CS of axial and extremity location by grade, followed by peripheral CS by grade and dedifferentiated CS by type.
DSS for all types by decade of diagnosis shows a tendency toward improved survival that does not reach significance (p = .09). At a subtype level, the DSS of peripheral and dedifferentiated CS remains unchanged. For central CS, those diagnosed 2010–2013 have a significant better survival than from both 1990–2000 and 2000–2010 which are similar (p = .03).
Prognostics
Univariate analysis is illustrated in Supplementary Table A.
shows the significant findings of multivariate analysis with hazard ratios and p-values following likelihood-ratio test for the cohort of central CS of bone excluding head and neck locations. Neither the distinction between extremity/axial location, nor size >8 cm predict behavior independantly in our analysis. Male patients have significant more local recurrence in this cohort and malignancy grade 3 independantly predicts risk of metastatis.
Table 4. Results of multivariate cox analysis; significant findings only.
Age at diagnosis and metastasis at diagnosis are both significant independent factors predicting DSS, while malignancy grade 3 has HR 2.91 (1.15-7.37) and p = .06 and does therefore not reach significance for DSS.
The presence of a soft-tissue component is an independent predictor of adverse levels of LR, Met and DSS.
Discussion
Norway is an ideal country for performing a national epidemiological study. The reporting of neoplasms and certain precancerous lesions to the NCR has been compulsory by law since 1951with a further registry act in 2002 requiring all hospitals, laboratory -staff and general practitioners to report all cases encountered. In addition to legislation, the registry is well resourced and has efficient track-back routines via the Norwegian Patient Registry. This has led to a completeness of reporting of 98.8% overall and of bone lesions specifically 97% [Citation34]. The Norwegian population is identifiable via an 11 digit social security number and has a stable sociopolitical system, limited migration and a comprehensive public health care system open to all inhabitants irrespective of means or income.
This is a retrospective study and its findings must be interpreted in consideration of this. As is the challenge for the sarcoma community in general, the level of evidence is low [Citation30] and there are to our knowledge no prospective studies of CS epidemiology. There are also large discrepancies in the quality of retrospective studies. This challenge is being met by expert panels creating statements to strengthen reporting. We have therefore attempted to comply with the ‘STROBE statement’ for the strengthening of observational studies as far as methodology allows.
To our knowledge, this is the first nationwide and complete report of CS epidemiology in a modern period. Although we have not been able to perform pathological review on all cases, we have attempted to review all those cases at risk of wrongful diagnosis or interpretation by review of clinical files for all patients and performed a full radiological review on over 70% of the cases. In total, we excluded 10% of the cases initially recognized and recorded as CS in the NCR. This strict inclusion together with quality control of all data and detail level strengthens our conclusions, but cannot eliminate the established interobserver variability involved in histological grading of chondroid lesions. The largest variability is in distinguishing grade 1 disease from benign, which justifids the exclusion of 11 borderline malignant cases and still revealed a grade 1 central extremity CS cohort without metastatic events. We have also chosen to exclude extremely rare subtypes like mesenchymal and clear cell CS, primarily to aid presentation of a complex cohort. The mesenchymal CS patients are presented in a previous EMSOS study [Citation35].
All our cases are histologically verified. This is not specified in SEER or NCIN publications. The SEER database includes 9.6% of soft tissue CS and reports a proportion of dedifferentiated CS of only 1.4% [Citation7]. The latter is broadly thought to represent approximately 10% of chondrosarcoma disease and represents 12.5% of our cohort.
This is the first registry study to report at a subtype level. Both UK and US data include CS from all sites and subtypes together. Numbers regarding CS as a whole are of little clinical use since we know that subtype, location and grade play a large part in depicting tumor behavior and expected rates of relapse. The subtypes of CS are increasingly recognized as separate diseases with differing etiology and biology [Citation36–38]. Peripheral CS has for example been shown to progress from a polarization at the epiphyseal growth plate. Central CS however has been postulated to arise from intramedullary stem cells [Citation39,Citation40]. One cannot presume significance on the CS group as a whole to be transferable to the more specific entities even though they have shared microscopic features.
We use the same systems of histological grading and staging for cartilaginous lesions today as in previous decades. Although not organized or published, there has been a definite change in this practice in the last 20–30 years based on the demonstrated significance of anatomical location, soft tissue components, growth pattern and subjectivity of assessment. There has also been a definite involvement of radiological features in the histological assessment since the early 1990s although this also has not been standardized. Our data involve only cases from a period when use of axial imaging was routine as opposed to the NCIN and SEER databases which report on cases from both the 1970s and 1980s where this was not in practice. We also report a shorter and more modern time era which should display more consistent clinical practice.
Recent work on the incidence of Osteosarcoma (OS) in Norway with similar methodology reported 3.8 per million/yr. for males; 2.8 for females and 3.3 for both genders combined for the time period 1975–2009 [Citation41]. Although the osteosarcoma incidence was fluctuating without clear time trends, our work reveals a clear increase and in fact, in the last period 2009–2013 an incidence of 3.45 per million/yr. for both sexes combined. This is similar to the incidence of OS described above.
Our found incidence of CS is quite high and higher than previously published as shown in . Our numbers are however in line with reporting to the IARC; where other Nordic countries like Finland, Denmark, and the Netherlands also report high levels of CS [Citation9] supporting the view that the external validity of this study is good.
Table 5. Comparison of incidence rates.
Histopathological assessment of chondroid tumors is prone to interobserver variability as mentioned previously [Citation42–44]. The highest level of variability concerns distinguishing benign disease from grade 1 disease. The increasing awareness of this could naturally result in a more careful practice and as such higher rates of reporting, particularly of grade 1 disease. In this material there is an increase in the number of grade 1 disease, but the increase in grade 2 is larger (), thereby supporting an actual increase rather than one related solely to changing nomenclature, definitions or more precautious practice. This variability exists for even expert sarcoma pathologist and definitely supports a strict exclusion of borderline pathology. It is possibly limited by integration of radiological features [Citation43] and as such our extensive radiological review is a likely strength though this has not been proven.
The review of this material has been performed by experienced tumor pathologists and radiologists, partly also in a group setting. They are however all involved in the clinical management of the very same population of patients and as such can be part of a systematic selection bias. A full external review was unfortunately beyond the scope and finances of this work but would have been preferential.
Soderstrom et al studied CS survival in 1970’s and 80’s in Finland without finding an improvement [Citation45]. This is likely due to the lack of developments in the effectiveness of adjuvant treatment for CS. There are however promising developments being made in understanding the role of the BCL-2 family of genes in CS chemoresistance. Routine clinical use of axial imaging was introduced in Oslo in the start of the 1990s. Although the role of surgery in itself probably is limited in being able to influence survival over time, the use of axial imaging could lead to earlier diagnosis, better selection of patients for differing surgical techniques and possibly better planning and obtained margins. This has been vital to limiting surgical morbidity as related to the safety of curettage for central intramedullary grade 1CS, reducing the extent of a ‘wide’ resection margin and possibly reducing the rates of contaminated surgery. Our findings of tendency for improved survival for all types and central CS specifically should however be interpreted with caution.
Current ESMO guidelines recommend follow-up for CS of bone for 10 years [Citation46]. Our data show that most local recurrences are discovered within 2 years and nearly all within 5 years, except for peripheral subtype. The actual rates are surprisingly stable at about 15%, again with the exception of grade 3 axial central and dedifferentiated disease. Rates of metastasis show a similar pattern with regard to when they become apparent although the frequency varies with subtype, grade and location. The knowledge that risk of recurrence or metastasis is minimal after a certain period of time should be of value for nervous patients and clinicians alike. Furthermore it can be used to tailor follow-up regimes with a higher level of evidence.
The 4th edition WHO classification of tumors refers to grade 1 CS and Atypical Cartilagenous Tumours (ACT) as synonyms [Citation33]. This should be based on an observed biology with negligible metastatic potential. In comparison, for highly differentiated lipomatous tumors the distinction between atypial lipomatous tumor (ALT) and highly differentiated liposarcoma grade 1 involves considering location; with ALT being used in the extremity only. From our data the very same distinction could be made for ACT’s with 0% 10-year metastatic rate in grade 1 central CS in the extremity but 11% for axial grade 1 central CS.
Although we report stable rates of recurrence and metastasis after 5 years, we can see that DSS continues to fall for numerous entities. This is likely an expression of inaccuracy in our variable “death from cancer”. This is natural with a low grade disease in a mostly elderly population. In the current cohort there are 59 reported 2nd cancers, six third cancers and even one fourth cancer listed in the NCR which can contribute this continued fall in DSS.
Prognostic analysis must be performed at a subtype level to be useful since biological aggressiveness varies widely between subtypes. Our analysis is intuitively in accordance with current clinical practice. Our finding of increased local recurrence in male patients is unusual and the reason unclear. It can of course be an incidencetal finding in a rare illness or be an expression of treatment selection bias by sex in this cohort. Metastasis at diagnosis is a strong predictor of adverse outcome as expected. Malignancy grade 3 is clearly a predictor of risk of metastasis and so thereby most likely also survival; though our model does not statistically confirm the latter.
Tumour size does not in any way seem to predict outcome. We publish here the categorical size variable defined by tumor size< >8cm, but have also tested < >10cm, < >15cm and size as a continuous variable. None of these variables reach significance in multivariate models.
The notion of increased aggressiveness of CS in axial versus extremity location is likely an expression of increased frequency of higher grade disease in axial disease rather than aggressiveness per grade according to analysis of this cohort, but warrants further investigation. Earlier published data have in large been bivariate in nature [Citation1,Citation2] and as such comparison is difficult. Age has been implicated previously; though as differing categorical variables rather than as a continuous such as ours. It is likely that our finding for age as nonsignificant for LR and Met but significant for DSS is an expression of inaccuracy in our DSS variable as mentioned above.
The presence of a soft tissue component is the only variable which reaches significance for all outcomes; local recurrence, metastasis and disease-specific survival. It has in the past been presented together with low and high malignancy grade and a distinction between extra- and intracompartmental disease as Enneking or AJCC stage [Citation1], but has not been included in multivariate analysis as a sole variable to date to our knowledge. This finding warrants further study to assess its reproducibility in other cohorts.
Conclusion
Our study found that the incidence of CS of bone in Norway appears to be increasing. In a modern setting; CS follows clear patterns of relapse and metastasis over time depending on subtype with malignancy grade 3 and the presence of a soft tissue component independently predicting behavior for the central CS subtype.
Research support
This project is in part funded by the Norwegian national advisory unit for Sarcoma.
Supplemental Material
Download MS Word (14.5 KB)Acknowledgments
This work has been made possible by the cooperation between tumor centers in Norway; making data and resources available in a positive and timely manner. Furthermore the availability and administration of the National Cancer Registry is a cornerstone for our work with a high level of professionalism. Tor Åge Myklebust; statistician at the NCR has also provided invaluable assistance and support in his proof-reading and advice concerning statistical analysis.
References
- Andreou D, Ruppin S, Fehlberg S, et al. Survival and prognostic factors in chondrosarcoma: results in 115 patients with long-term follow-up. Acta Orthop. 2011;82:749–755.
- Angelini A, Guerra G, Mavrogenis AF, et al. Clinical outcome of central conventional chondrosarcoma. J Surg Oncol. 2012;106:929–937.
- Bjornsson J, McLeod RA, Unni KK, et al. Primary chondrosarcoma of long bones and limb girdles. Cancer. 1998;83:2105–2119.
- Fiorenza F, Abudu A, Grimer RJ, et al. Risk factors for survival and local control in chondrosarcoma of bone. J Bone Joint Surg Br. 2002;84:93–99.
- Pritchard DJ, Lunke RJ, Taylor WF, et al. Chondrosarcoma: a clinicopathologic and statistical analysis. Cancer. 1980;45:149–157.
- Anfinsen KP, Devesa SS, Bray F, et al. Age-period-cohort analysis of primary bone cancer incidence rates in the United States (1976-2005). Cancer Epidemiol Biomarkers Prev. 2011;20:1770–1777.
- Giuffrida AY, Burgueno JE, Koniaris LG, et al. Chondrosarcoma in the United States (1973 to 2003): an analysis of 2890 cases from the SEER database. J Bone Joint Surg Am. 2009;91:1063–1072.
- Whelan J, McTiernan A, Cooper N, et al. Incidence and survival of malignant bone sarcomas in England 1979-2007. Int J Cancer. 2012;131:E508–E517.
- Valery PC, Laversanne M, Bray F. Bone cancer incidence by morphological subtype: a global assessment. Cancer Causes Control. 2015;26:1127–1139.
- Rizzo M, Ghert MA, Harrelson JM, et al. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res. 2001;391:224–233.
- Lee FY, Mankin HJ, Fondren G, et al. Chondrosarcoma of bone: an assessment of outcome. J Bone Joint Surg Am. 1999;81:326–338.
- Donati D, El Ghoneimy A, Bertoni F, et al. Surgical treatment and outcome of conventional pelvic chondrosarcoma. J Bone Joint Surg Br. 2005;87:1527–1530.
- Jennings R, Riley N, Rose B, et al. An evaluation of the diagnostic accuracy of the grade of preoperative biopsy compared to surgical excision in chondrosarcoma of the long bones. Int J Surg Oncol. 2010;2010:270195.
- Bauer HC, Brosjo O, Kreicbergs A, et al. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities. 80 patients followed for 2–25 years. Acta Orthop Scand. 1995;66:283–288.
- Campanacci DA, Scoccianti G, Franchi A, et al. Surgical treatment of central grade 1 chondrosarcoma of the appendicular skeleton. J Orthopaed Traumatol. 2013;14:101–107.
- Di Giorgio L, Touloupakis G, Vitullo F, et al. Intralesional curettage, with phenol and cement as adjuvants, for low-grade intramedullary chondrosarcoma of the long bones. Acta Orthop Belg. 2011;77:666–669.
- Hickey M, Farrokhyar F, Deheshi B, et al. A systematic review and meta-analysis of intralesional versus wide resection for intramedullary grade I chondrosarcoma of the extremities. Ann Surg Oncol. 2011;18:1705–1709.
- Mohler DG, Chiu R, McCall DA, et al. Curettage and cryosurgery for low-grade cartilage tumors is associated with low recurrence and high function. Clin Orthop Relat Res. 2010;468:2765–2773.
- Schreuder HW, Pruszczynski M, Veth RP, et al. Treatment of benign and low-grade malignant intramedullary chondroid tumours with curettage and cryosurgery. Eur J Surg Oncol. 1998;24:120–126.
- Souna BS, Belot N, Duval H, et al. No recurrences in selected patients after curettage with cryotherapy for grade I chondrosarcomas. Clin Orthop Relat Res. 2010;468:1956–1962.
- Streitburger A, Ahrens H, Balke M, et al. Grade I chondrosarcoma of bone: the Münster experience. J Cancer Res Clin Oncol. 2009;135:543–550.
- Bernard SA, Murphey MD, Flemming DJ, et al. Improved differentiation of benign osteochondromas from secondary chondrosarcomas with standardized measurement of cartilage cap at CT and MR imaging. Radiology. 2010;255:857–865.
- Brenner W, Conrad EU, Eary JF. FDG PET imaging for grading and prediction of outcome in chondrosarcoma patients. Eur J Nucl Med Mol Imaging. 2004;31:189–195.
- Douis H, Singh L, Saifuddin A. MRI differentiation of low-grade from high-grade appendicular chondrosarcoma. Eur Radiol. 2014;24:232–240.
- Geirnaerdt MJ, Bloem JL, Eulderink F, et al. Cartilaginous tumors: correlation of gadolinium-enhanced MR imaging and histopathologic findings. Radiology. 1993;186:813–817.
- Geirnaerdt MJ, Hermans J, Bloem JL, et al. Usefulness of radiography in differentiating enchondroma from central grade 1 chondrosarcoma. AJR Am J Roentgenol. 1997;169:1097–1104.
- Geirnaerdt MJ, Hogendoorn PC, Bloem JL, et al. Cartilaginous tumors: fast contrast-enhanced MR imaging. Radiology. 2000;214:539–546.
- Berber O, Datta G, Sabharwal S, et al. The safety of direct primary excision of low-grade chondral lesions based on radiological diagnosis alone. Acta Orthop Belg. 2012;78:254–262.
- Vanel D, Kreshak J, Larousserie F, et al. Enchondroma vs. chondrosarcoma: a simple, easy-to-use, new magnetic resonance sign. Eur J Radiol. 2013;82:2154–2160.
- Evaniew N, Nuttall J, Farrokhyar F, et al. What are the levels of evidence on which we base decisions for surgical management of lower extremity bone tumors?. Clin Orthop Relat Res. 2014;472:8–15.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349.
- Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977;40:818–831.
- Fletcher CD, Bridge JA, Hogendoorn PC, et al. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: International Agency for Research on Cancer (IARC); 2013.
- Larsen IK, Smastuen M, Johannesen TB, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45:1218–1231.
- Frezza AM, Cesari M, Baumhoer D, et al. Mesenchymal chondrosarcoma: prognostic factors and outcome in 113 patients. A European Musculoskeletal Oncology Society study. Eur J Cancer. 2015;51:374–381.
- Bovee JV, Cleton-Jansen AM, Kuipers-Dijkshoorn NJ, et al. Loss of heterozygosity and DNA ploidy point to a diverging genetic mechanism in the origin of peripheral and central chondrosarcoma. Genes Chromosom Cancer. 1999;26:237–246.
- Bovee JV, van den Broek LJ, Cleton-Jansen AM, et al. Up-regulation of PTHrP and Bcl-2 expression characterizes the progression of osteochondroma towards peripheral chondrosarcoma and is a late event in central chondrosarcoma. Lab Invest. 2000;80:1925–1934.
- de Andrea CE, Hogendoorn PC. Epiphyseal growth plate and secondary peripheral chondrosarcoma: the neighbours matter. J Pathol. 2012;226:219–228.
- Douis H, Davies AM, James SL, et al. Can MR imaging challenge the commonly accepted theory of the pathogenesis of solitary enchondroma of long bone? Skeletal Radiol. 2012;41:1537–1542.
- Aigner T. Towards a new understanding and classification of chondrogenic neoplasias of the skeleton-biochemistry and cell biology of chondrosarcoma and its variants. Virchows Arch. 2002;441:219–230.
- Berner K, Johannesen TB, Berner A, et al. Time-trends on incidence and survival in a nationwide and unselected cohort of patients with skeletal osteosarcoma. Acta Oncol. 2015;54:25–33.
- de Andrea CE, Kroon HM, Wolterbeek R, et al. Interobserver reliability in the histopathological diagnosis of cartilaginous tumors in patients with multiple osteochondromas. Mod Pathol. 2012;25:1275–1283.
- Eefting D, Schrage YM, Geirnaerdt MJ, et al. Assessment of interobserver variability and histologic parameters to improve reliability in classification and grading of central cartilaginous tumors. Am J Surg Pathol. 2009;33:50–57.
- Skeletal Lesions Interobserver Correlation among Expert Diagnosticians (SLICED) Study Group. Reliability of histopathologic and radiologic grading of cartilaginous neoplasms in long bones. J Bone Joint Surg Am. 2007;89:2113–2123.
- Soderstrom M, Ekfors TO, Bohling TO, et al. No improvement in the overall survival of 194 patients with chondrosarcoma in Finland in 1971-1990. Acta Orthop Scand. 2003;74:344–350.
- The ESMO/European Sarcoma Network Working Group. Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25:iii113–iii123.