Abstract
Background: Cancer and cancer treatments may impact the brain through several pathways leading to cognitive impairment. Neuroimaging evidence has begun to elucidate the neurobiological underpinnings of cancer-related cognitive impairment. The aim of this paper was to systematically review available literature on structural brain alterations following adult non-central nervous system (CNS) cancers and associated treatments.
Methods: This review followed PRISMA guidelines and was registered in PROSPERO (ID#107387). Comprehensive searches were conducted in June 2018 using PubMed and Web of Science. Inclusion criteria were English peer-reviewed journal articles of formal, controlled studies that examined structural neuroimaging outcomes in adult non-CNS cancer patients and survivors. Selected articles were assessed for quality and risk of bias using the National Institutes of Health Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies.
Results: Thirty-six publications of prospective and cross-sectional studies met inclusion criteria and were included. Structural brain alterations following cancer and its treatment were reported in a majority of the publications as evidenced by reduced global and local gray matter volumes, impaired white matter microstructural integrity, and brain network alterations. Structural alterations were most often evident when cancer-treated groups were compared with healthy controls, and more subtle when compared with cancer controls. Regarding the existence of pretreatment impairments, the evidence was equivocal. There was significant between-study heterogeneity in imaging analytical approaches and use of statistical adjustments. Over half reported associations with cognitive outcomes, though regions and associated cognitive domains were heterogeneous.
Conclusions: Structural brain alterations following cancer and cancer treatments were reported in a majority of the reviewed studies. However, the extent of observed alterations depended on the choice of comparison groups. Methodological issues exist that will need to be addressed systematically to ensure the validity of findings. Large-scale prospective studies with extended assessment points are warranted to replicate and build upon initial findings.
Background
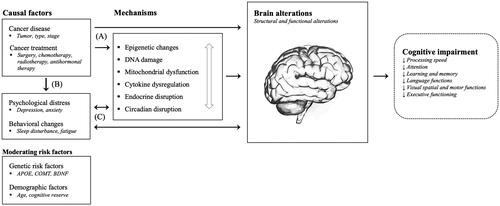
Converging evidence indicates that cancer and cancer treatment is associated with cognitive impairment in patients with non-central nervous system (CNS) cancers [Citation1]. Initially called ‘chemo-brain’, the term cancer-related cognitive impairment (CRCI) has since been adopted by researchers, as it more accurately captures its complex etiology, which may not uniquely be attributed to neurotoxicity caused by chemotherapy. A body of research has emerged suggesting that cognitive impairment may be evident prior to the initiation of systemic therapies pointing to the cancer itself as a potential causal factor [Citation2]. Other co-occurring symptoms such as fatigue, sleep, and mood disturbances may also contribute to CRCI. Research examining the underlying pathophysiological mechanisms of CRCI has identified several candidate mechanisms. A dominant model relates to the role of proinflammatory cytokines [Citation3]. The release of these inflammation-promoting signaling molecules (e.g., interleukin-6) can be triggered directly by the cancer and tumor growth, as a secondary process related to local and systemic treatments, or as a consequence of altered behavioral and psychological factors [Citation4,Citation5]. Once released, cytokines can signal the brain through several pathways, leading to alterations in neurotransmitter function and brain circuitry [Citation6]. Other candidate mechanisms of CRCI include DNA damage and oxidative stress, telomere shortening, mitochondrial dysfunction, epigenetic changes, as well as, endocrine and circadian disruption [Citation7–10]. These pathophysiological mechanisms of CRCI should not be regarded as competing explanatory models, but as co-occurring and dependent processes that may lead to CRCI (). Furthermore, emerging research on moderating risk factors including cognitive reserve and specific genetic predispositions suggest that some patients may be at an increased risk [Citation11].
Figure 1. Several pathways are hypothesized to underlie the detrimental impact of cancer and cancer treatments on the brain and cognitive functions. First, cancer and cancer treatments (e.g., chemotherapy) may either directly, or indirectly through various pathophysiological mechanisms including epigenetic changes, DNA damage and oxidative stress, mitochondrial dysfunction, pro-inflammatory cytokine release, and endocrine and circadian disruptions, result in brain alterations and cognitive impairment (A). These mechanisms should be regarded as co-occurring and dependent processes as indicated by the white arrow. Second, cancer and cancer treatments may lead to increased psychological distress (e.g., symptoms of depression and anxiety) and behavioral changes (e.g., sleep disturbances), which may again, either directly or indirectly, impact the brain and cognitive functions (B+C). Third, activated mechanisms and associated brain alterations, as well as cognitive changes, may on their own have a negative impact on psychological and behavioral factors resulting in a negative feedback loop (C). Finally, known genetic and demographic risk factors may moderate these pathways.
Irrespective of the exact underlying pathophysiology, it must be assumed that CRCI is mediated by brain alterations. Indeed, there is emerging neuroimaging research elucidating the underlying neurobiological basis of CRCI. The neuroimaging literature can be categorized into functional and structural approaches. Both approaches have been adopted within CRCI research as they provide answers to different questions. While functional studies rely on the in vivo assessment of ongoing brain activity at rest or during specific tasks to investigate potentially altered brain activation patterns following cancer and cancer treatments, structural approaches rely on the quantification of anatomical, morphological, and microstructural properties of the physical brain, most commonly measured in white matter (WM) and gray matter (GM) tissue, to investigate potentially altered structural properties of the brain related to cancer and its treatment. Central structural imaging modalities include T1-weighted magnetic resonance imaging (MRI) and diffusion-weighted imaging (referred to as diffusion tensor imaging, DTI). T1-weighted MRI allows for high resolution anatomical images with excellent contrast between WM and GM. It is useful for morphometric and volumetric analysis of manually or automatically delineated region of interests (ROIs) (e.g., the hippocampus). More recent developments include fully automated approaches such as voxel-based morphometry (VBM) [Citation12], which employs voxel-wise parametric statistical testing of GM density across the entire brain or in specified ROIs. DTI is another MRI technique that uses the directional coherence of water diffusion in the brain. Due to the uniformity of the fibrous structure of WM, DTI can be used to indirectly assess the directionality and microstructural integrity of WM tracts. Common DTI measures are fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). These measures can be analyzed using a variety of approaches including voxel and tract-based analysis, ROI analysis, and network analysis.
In line with this, the aim of the present paper was to systematically and comprehensively review the structural neuroimaging literature in order to answer the following questions: Is cancer and cancer treatment associated with structural brain alterations in adult cancer patients with non-CNS cancers? Are there differences in structural brain alterations between cancer patients who receive treatment compared with appropriate controls?
Methods
Registration and data source
The present systematic review was registered in The International Prospective Register of Systematic Reviews (PROSPERO) under ID# 107387 and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation13]. A comprehensive literature search of PubMed and Web of Science was undertaken on 8 June 2018. Data were extracted from studies published in peer-reviewed journals using structural neuroimaging in adult non-CNS cancer patients (see ). For details on study eligibility, search strategy, quality assessment, and data extraction, see the Supplementary material.
Results
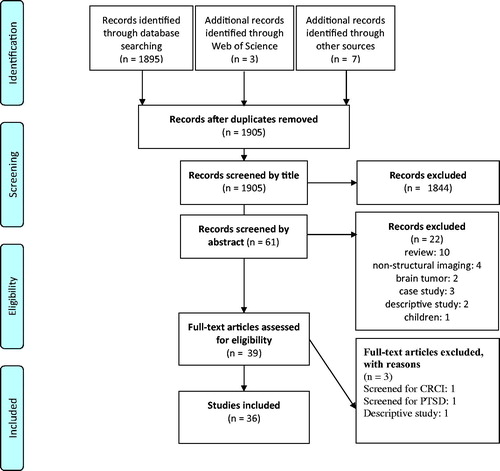
Flowchart of the selection process is presented in . Summary data and results extracted from the articles are presented in and .
Table 1. Main study characteristics.
Table 2. Results by the comparison group.
Characteristics of the included studies
A total of 36 publications were included in this systematic review [Citation14–49]. Below, we describe the main study characteristics.
Cancer diagnoses
Twenty-eight publications were focused on breast cancer (BC) patients [Citation14–41], three focused on testicular cancer patients [Citation42–44], two on patients undergoing hematopoietic stem cell transplant [Citation45,Citation46], one on lung cancer patients [Citation47], one on prostate cancer patients [Citation48], and one on ovarian, peritoneal, and fallopian tube cancer patients [Citation49]. Five of the BC publications [Citation15,Citation17,Citation21,Citation24,Citation40] were associated with two research projects and six additional BC publications [Citation14,Citation22,Citation23,Citation30,Citation32,Citation39] were connected with three research projects with overlapping samples. Inagaki et al. [Citation30] reported findings from two distinct samples and were counted separately in . Two testicular cancer publications were from one research project [Citation42,Citation44], as were the two publications pertaining to patients undergoing hematopoietic stem cell transplant [Citation45,Citation46].
Sample size
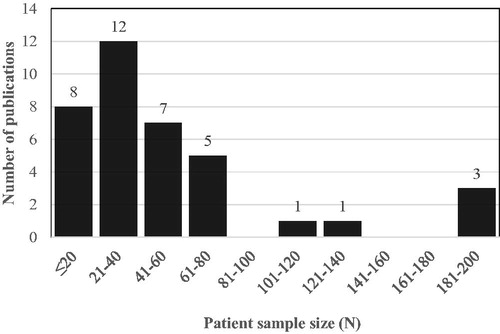
Median patient sample size for the 36 reviewed studies was in the 21–40 participant range. Four publications included sample sizes exceeding 100 (from two projects) [Citation17,Citation24,Citation30,Citation40], but the remaining studies had patient sample sizes that were less than 80, see .
Study design
Of the 28 BC studies, 19 were cross-sectional [Citation14,Citation17,Citation19,Citation22–25,Citation27,Citation28,Citation30–34,Citation36,Citation37,Citation39–41] and nine were longitudinal designs [Citation15,Citation16,Citation18,Citation20,Citation21,Citation26,Citation29,Citation35,Citation38]. One of the testicular cancer studies was cross-sectional [Citation43], and the remaining two were longitudinal [Citation42,Citation44]. The lung cancer [Citation47] and ovarian cancer studies [Citation49] were cross-sectional. The two hematopoietic stem cell transplant studies [Citation45,Citation46] and the prostate cancer study [Citation48] were longitudinal.
Cancer treatment
Four studies focused specifically on chemotherapy-naïve BC patients during or post-surgery [Citation16,Citation25,Citation37,Citation41]; 28 studies focused on cancer patients who were undergoing/had undergone chemotherapy [Citation14,Citation15,Citation17–24,Citation26–32,Citation34–36,Citation38–40,Citation42–44,Citation47,Citation49]; two studies focused on hematopoietic stem cell transplant patients [Citation45,Citation46]; and two studies focused on patients undergoing antihormonal treatment [Citation33,Citation48].
Imaging modalities and brain structures
All of the identified studies used MRI as the structural imaging technique. Analysis of T1-weighted data was reported in 27 publications, while 15 studies reported on DTI data with several studies reporting both. Structural assessment of GM was reported in a total of 21 publications (58%) [Citation14,Citation16,Citation18–20,Citation22–26,Citation29,Citation30,Citation37,Citation40,Citation41,Citation43,Citation44,Citation46–49] with VBM being the most widely used method of analysis (n = 17; 81%). Two studies used whole-brain network analyses to assess GM covariance networks [Citation23,Citation37]. One study assessed overall lobe volumes [Citation26], and one study quantified cerebral pathology [Citation40]. Structural assessment of the brain WM was reported in a total of 20 publications (56%) [Citation14,Citation15,Citation17,Citation21,Citation22,Citation24–26,Citation28,Citation30,Citation31,Citation35,Citation38–43,Citation45,Citation47]. The most widely used imaging technique to assess the WM structure was diffusion-weighted imaging. Voxel-wise, tract-based, and ROI analyses using one or several diffusion metrics (i.e., FA, MD, AD, and RD) was reported in 14 publications [Citation14,Citation15,Citation17,Citation21,Citation22,Citation28,Citation31,Citation35,Citation38,Citation39,Citation41,Citation43,Citation45,Citation47]. Results from volumetric analyses were reported in five publications [Citation24–26,Citation30,Citation43], while two publications reported results from whole-brain network analysis [Citation39,Citation42].
Quality rating of studies and risk of bias
Quality assessment of each study was undertaken as described in the Supplementary material. Ten studies were deemed good quality (>9 criteria met) [Citation14,Citation16,Citation22,Citation27,Citation35,Citation37,Citation40,Citation42–44] and 26 were deemed fair quality (5–9 criteria met) [Citation15,Citation17–21,Citation23–26,Citation28–34,Citation36,Citation38,Citation39,Citation41,Citation45–49]. All studies were included in our results. The quality rating process highlighted areas of weakness that may increase risk of bias. With respect to recruitment and accruals reporting, most studies did not include the dates during which data collection occurred, about 20% of studies did not include the location of participant recruitment, and 10% provided no information about how HC participants were recruited. About half of the studies either did not report follow-up rates or had drop-out rates greater than 20%. Although most studies included information about how they undertook the matching of controls to the primary patient sample(s), approximately 30% of the studies did not specify any matching procedure beyond the general inclusion and exclusion criteria. With respect to data analysis, no study provided a priori sample size justification or discussion of power, but 50% did provide sufficient information to calculate effect sizes for potential meta-analyses.
With respect to the imaging data, scan acquisitions within each study were most commonly undertaken using the same scanner, and acquisition parameters were held constant across participants. Between-study variability in acquisition parameters, however, were noted. Post-processing was largely conducted using automated and standardized pipelines, although study variability did exist. Regarding the imaging analyses, most studies used standardized and well-known approaches (e.g., VBM, tract-based spatial statistics). Furthermore, while the majority of studies undertook some form of multiple comparison adjustments such as family-wise error and false discovery rate corrections or corrections for cluster level thresholds in voxel-level analyses, large between-study heterogeneity existed in the choice of parameters. In the 10 studies that included some form of manual assessment or quality check of the imaging data (i.e., delineation of hippocampal volumes, WM lesions, and small-vessel disease) [Citation14,Citation22,Citation27,Citation31–34,Citation40,Citation41,Citation43], three did not explicitly report blinding raters to group condition [Citation32,Citation40,Citation43]. Two studies used multiple raters in order to measure inter-rater reliability, which was determined to be high [Citation32,Citation33]. Most studies stated research questions or hypotheses and defined their inclusion and exclusion criteria adequately. Importantly, studies generally defined their outcome variables clearly and used valid and reliable approaches to assess structural properties. Most studies included important covariates and, if they had not, had matched their samples on characteristics important to the outcome variables (e.g., age).
Structural alterations related to cancer and its treatment
Results regarding the association between brain structural alterations and cancer and its treatment will be presented according to the main treatment modality investigated (e.g., post-surgery, chemotherapy, antihormonal therapy). When applicable, results are then organized by cancer type within each treatment modality (e.g., breast, testicular, etc.).
Surgery/pre-chemotherapy
Four studies were identified that specifically investigated the impact of surgery and anesthesia [Citation16] and cancer itself on structural brain properties [Citation25,Citation37,Citation41]. Sato et al. [Citation16] prospectively compared GM density in 32 postmenopausal BC patients undergoing surgery with 20 age-matched healthy controls (HCs). In accordance with a priori hypotheses, they found significant reductions across time in GM density in the right thalamus of BC patients compared with HCs. Using DTI, Menning et al. [Citation41] cross-sectionally compared BC patients who were scheduled or not scheduled for chemotherapy with HCs and found distributed areas with altered WM among patients, though differences were no longer statistically significant when controlling for fatigue levels. In addition, regional GM and WM volumes were not significantly different between groups. Scherling et al. [Citation25] also examined GM volumes by comparing 23 BC patients prior to chemotherapy with 23 matched controls and found no between-group differences. Lower WM volumes, however, were observed in frontal, parietal, and limbic regions in the patients. Using network analysis, Kesler et al. [Citation37] compared GM covariance networks of BC patients post-surgery with HCs and found evidence of altered local clustering in frontal, parietal, and temporal regions, but not globally. Baseline results from longitudinal studies of both GM volumes and WM microstructure also elucidated potential post-surgery brain structural alterations. In one study, no difference in GM volume was detected between BC patients awaiting chemotherapy and those who were not or HCs [Citation29]. Another study by the same group, however, reported lower GM volumes in the left cingulate gyrus in patients who did not subsequently receive chemotherapy compared with HCs, but no differences were noted with those who went on to receive chemotherapy [Citation20]. Regarding WM, Deprez et al. [Citation21] found no difference in WM microstructure between premenopausal BC patients and HCs at baseline prior to chemotherapy.
Chemotherapy
Publications in breast cancer (BC): A total of 23 publications (64%) investigated the association between chemotherapy and structural brain alterations in BC populations. Of these, 15 (65%) were cross-sectional [Citation14,Citation17,Citation19,Citation22–24,Citation27,Citation28,Citation30–32,Citation34,Citation36,Citation39,Citation40] and eight (35%) were longitudinal [Citation15,Citation18,Citation20,Citation21,Citation26,Citation29,Citation35,Citation38]. Three publications were effectively null findings [Citation26,Citation32,Citation38] while the remainder reported associations between chemotherapy and structural brain alterations.
Cross-sectional findings: Of the cross-sectional studies, 10 compared patients who received chemotherapy (CT+) with a HC group as the only comparison condition [Citation17,Citation19,Citation23,Citation24,Citation27,Citation31,Citation34,Citation36,Citation39,Citation40]. Three studies included a non-CT cancer control group (CT−) in addition to a HC group [Citation14,Citation28,Citation30], while two studies compared CT + with CT − only [Citation22,Citation32]. In studies comparing CT + patients with HCs, CT + evidenced structural brain alterations including reduced hippocampal volumes and deformation [Citation27,Citation34,Citation36]; long-term reductions in regional and global GM and VM volumes [Citation17,Citation19,Citation24]; altered brain structural networks [Citation23,Citation39]; as well as lower WM integrity [Citation28,Citation31]. A higher prevalence of cerebral microbleeds was also found, but the result would not have survived adjustment for multiple comparisons [Citation40]. Although one study in BC patients who were an average of 6 years post-CT found that time since chemotherapy was positively correlated with GM density [Citation19], studies that included BC survivors 21 years post-treatment still found structural impairments in multiple areas [Citation17,Citation24,Citation40]. In contrast, other studies that compared CT + patients with HCs, did not detect differences in GM or WM 1 and 3 years post-treatment [Citation30]. In studies that compared CT + with CT−, most found that CT + patients evidenced structural alterations as indicated by: reductions in GM volumes in posterior regions [Citation14,Citation22], reductions in both GM and WM in frontal and temporal regions [Citation30]; and impaired widespread microstructural integrity of the WM [Citation14,Citation22,Citation28]. Again, some of these impairments were apparent 10 years post-treatment [Citation14,Citation22]. One study did not observe differences in hippocampal volumes between CT + and CT − patients [Citation32].
Longitudinal findings: Eight prospective studies with BC patients were identified of which four investigated GM changes [Citation18,Citation20,Citation26,Citation29] and four focused on WM microstructural changes [Citation15,Citation21,Citation35,Citation38]. Regarding changes in GM volumes, two studies followed patients from post-surgery but prior to further treatment with subsequent follow-up at 1 month and 1 year post-treatment [Citation18,Citation29]. In these studies, within-group analysis revealed widespread bilateral GM reductions from baseline to 1 month after treatment pertaining to frontal and temporal regions. Time-by-group interactions, however, revealed fewer significant clusters, and it is worth noting that in the study by Lepage et al. [Citation18], statistical comparisons were restricted to within-group differences only, with no formal test of an interaction. Within-group analyses from baseline to 1-year post-treatment in these studies revealed partial recovery in multiple regions including the temporal lobe. Persistent bilateral reductions were observed in frontal and cerebellar regions. In a prospective replication study by McDonald et al. [Citation20], within-group GM reductions from baseline to 1 month after chemotherapy was observed in frontal regions in CT + patients. A group-by-time interaction revealed specific reduction in the left middle frontal gyrus, which replicated earlier findings. With respect to microstructural WM alterations, Deprez et al. [Citation21] reported within-group reductions in FA in frontal, parietal, and occipital WM regions in CT + patients. No changes were observed in CT − or HC groups. In a follow-up study of the same cohort, patients were reassessed after 3–4 years [Citation15]. Restricting their analysis to previously impaired WM regions, the results indicated a recovery back to baseline levels. A recent prospective study comparing 26 CT + with 23 CT- patients and 30 HCs at baseline, and at a six months follow-up, found no changes in WM microstructure (FA/MD) in either group [Citation35]. ROI analyses, however, revealed changes in the superior longitudinal fasciculus fiber tract with more pronounced decline in FA in the CT + group compared with CT−. Interestingly, no difference was observed when compared with HCs. Two prospective studies did not find significant changes in regional or global GM and WM volumes between HC and CT+ [Citation26], nor in regional microstructural properties [Citation38].
Publications in testicular cancer (TC): To date, results on the association between CT and brain structural properties in TC come from three publications [Citation42–44]. One cross-sectional study investigated the long-term effects of cisplatin-based CT on GM/WM volumes and WM microstructure [Citation43]. Compared with CT−, CT + evidenced widespread increase in radial kurtosis, but not in other diffusion parameters (i.e., FA, MD). No between-group differences were observed in global or focal GM or WM volumes. Two prospective studies from the same project investigated changes in GM volumes and WM networks in CT + compared with CT− [Citation42,Citation44]. Assessing CT + and CT − patients at baseline following orchiectomy and six months after, corresponding to 3 months post-CT, Amidi et al. [Citation44] found significant reductions in frontal GM volumes across time in CT+. Within-group analyses revealed widespread bilateral loss of GM in both groups in frontal, parietal, and occipital regions. A subsequent study of the same patient cohort further revealed changes in the structural brain network in the CT + group relative to CT − as indicated by decreased small-worldness, networking clustering, and local efficiency [Citation42].
Publications in hematopoietic stem cell transplant (HSCT) patients: Two publications from one longitudinal project examined structural brain alterations in HSCT patients [Citation45,Citation46]. HSCT is an established treatment for many hematological malignancies involving an intensive conditioning regimen consisting of high-dose CT with or without total body irradiation followed by infusion of either a donor’s (allogeneic) or the patient’s own (autologous) stem cells [Citation50]. Pre-transplant, there were no differences found between HCs and patients in regional brain volume, lateral ventricle volume or WM integrity [Citation45,Citation46]. However, allogeneic HSCT candidates had higher MD and AD in the left hemisphere compared with autologous candidates’ pre-transplant [Citation45]. In longitudinal analyses, patients showed GM reductions in the middle frontal gyrus bilaterally and in the left caudate nucleus, increases in left lateral and total ventricle volume, and a significant decrease in MD and AD in diffuse WM regions relative to HCs from baseline to one year post-HSCT [Citation45,Citation46]. Differences were also found by transplant type; 1 year post-HSCT, allogeneic HSCT recipients had lower FA and higher RD in the right hemisphere and left frontal WM compared to autologous recipients.
Other cancer populations: There has been one publication on structural brain alterations in lung cancer patients. Simó et al. [Citation47] cross-sectionally compared 28 small-cell lung cancer patients after CT with 20 matched chemo-naïve non-small-cell lung cancer patients, and 20 HCs. Their results revealed lower GM within the temporal, parietal, and frontal regions in the CT + group relative to HCs. Compared with HCs, both patient groups evidenced impaired WM microstructure bilaterally in inferior longitudinal fasciculus and the left cingulum. No differences were observed in either WM or GM properties between patient groups (CT + versus CT−). One study has been published on structural impairments in 18 ovarian, peritoneal and fallopian cancer patients. Patients who had completed CT within 1–4 months were compared with 18 matched HCs [Citation49]. Lower GM volumes in patients were observed in frontal and parietal regions including in the right frontal gyrus, left frontal operculum and left supramarginal gyrus.
Antihormonal therapies
Two identified publications specifically investigated the association between antihormonal treatment and brain structural alterations [Citation33,Citation48]. In a prospective study by Chao et al. [Citation48], GM density in 12 prostate cancer patients initiating androgen deprivation therapy (ADT) was compared with 12 matched non-ADT prostate cancer patients. Decreased GM was observed from baseline to 6 months after ADT in the primary motor cortex and the dorsolateral prefrontal cortex relative to the non-ADT group. Eberling et al. [Citation33] examined three groups of postmenopausal women – women taking estrogen, women with BC taking tamoxifen, and women not taking estrogen or tamoxifen. Hippocampal volumes in BC patients were not different from volumes in women not taking estrogen or tamoxifen.
Association between structural neuroimaging outcomes and cognitive functions
Twenty-eight publications examined correlates with cognitive outcomes using neuropsychological tests [Citation14–16,Citation18,Citation19,Citation21,Citation22,Citation25,Citation26,Citation28,Citation30–42,Citation44–46,Citation48,Citation49]. In most cases, studies reported associations between regions of interest or those that differed between groups/changed over time with neuropsychological outcomes, or vice versa. Several studies found significant correlations between brain structures and various cognitive outcomes, particularly in those who received cancer treatment. Significant findings were expressed as associations between reduced GM density, poorer WM integrity, more WM lesions, smaller hippocampal volume, or less efficient brain networks with impaired cognitive performance typically in one or two domains in the cancer-treated patient group, or across the whole sample [Citation14,Citation18,Citation19,Citation21,Citation28,Citation30,Citation31,Citation34,Citation39,Citation40,Citation42,Citation44,Citation45,Citation48]. See Supplementary Table 1 for a summary of these results.
Discussion
The aim of this systematic review was to summarize findings on the association between cancer and cancer treatment, and structural brain alterations. We believe that the present paper represents the most comprehensive review to date on this topic. Thirty-six publications were identified, of which the majority included BC populations. Other cancer types were testicular, hematologic, ovarian, and lung cancers.
All of the studies were of fair to good quality but areas were also identified that could increase the risk of bias within studies and overall. For the most part, however, efforts were made to undertake appropriate statistical controls, such as multiple comparison adjustments, inclusion of relevant covariates, and the use of reliable approaches in imaging post-processing and data analysis.
Summarizing the results, it is clear that structural brain alterations were reported in a majority of the studies and included evidence of reduced global and local GM volumes, impaired microstructural WM integrity, and brain network alterations. A majority found evidence for lower GM density in cancer patients when compared with HCs, and in patients following systemic treatment compared with cancer controls in at least one or more brain regions. One of the largest cross-sectional studies to date found significantly lower total brain and GM volumes in chemotherapy-exposed patients [Citation24]. Affected GM regions, however, varied by study with no clear pattern. Studies that attempted to elucidate treatment-specific changes controlled for both cancer-treatment and cancer through the inclusion of at least one cancer control group and a HC group. However, such studies were generally less able to detect GM alterations, particularly between cancer groups, potentially due to small sample sizes. One aberration was the relatively larger study by Inagaki et al. [Citation30] where GM differences between CT − and CT + groups were detected. Studies that tried to distinguish impact on GM due to surgery or the cancer itself, also culminated in equivocal findings. Furthermore, time since treatment did not appear to mitigate the effect, at least in BC patients. Although partial short-term recovery in GM was found following chemotherapy treatment [Citation18,Citation29], long-term BC survivors evidenced reduced GM [Citation14,Citation19,Citation22,Citation24]. This could, however, be due to historical differences in treatment regimens and doses.
Regarding structural impairments in WM, results from volumetric studies were generally inconsistent. However, all studies that used DTI to assess WM microstructure, except one [Citation17], revealed alteration in one or more diffusion measures such as reduced FA indicative of lower structural integrity of WM fiber tracts, and increased MD values. The advantage of diffusion-weighted imaging is that it is a non-invasive, yet highly sensitive technique to detect WM abnormalities [Citation51]. Again, the most consistent findings were observed when comparing CT + with HC. However, several studies also reported differences between treatment groups (e.g., CT + versus CT−) in both cross-sectional and longitudinal studies with indications of lower WM integrity in CT+. Overall, DTI appeared to be a sensitive technique for the detection of acute cancer and treatment-related effects on WM, not only in relation to chemotherapy in BC patients but also in relation to other types of treatments and cancers. Regarding the pattern of WM alterations, results were less consistent with evidence of widespread and diffuse impairments, suggesting that the effect of cancer and its treatment on WM microstructure does not carry a signature pattern but is widespread and diffuse. When assessed several years after treatment, alterations in the brain WM were generally not evident, potentially indicating long-term recovery in the WM structure. Direct longitudinal evidence of recovery following chemotherapy for BC was reported in one of the few studies that included a long-term follow-up assessment [Citation15]. In contrast, evidence also indicated long-term WM abnormalities in BC patients who had received a high dose of chemotherapy [Citation22]. These DTI findings, however, should be interpreted with caution due to several issues. First, heterogeneity was observed regarding how many and which diffusion metrics were included. Most commonly, FA was used as a measure of WM integrity, but several studies included multiple diffusion measures that may have increased the risk of Type I error due to multiple testing. Second, studies found changes in some diffusion metrics but not in others. Third, while different DTI measures may indicate different types of WM abnormalities not captured by FA, the clinical interpretation is complex and should be performed with care [Citation52]. In the absence of clearly stated a priori hypotheses, such findings may have represented selective reporting. Finally, the mode of analysis employed differed between studies with some opting for whole-brain voxel-wise comparisons, while others restricted their analysis to tract-based techniques, and yet others employed ROI-based methods. Other general considerations relate to the image acquisition parameters such as the strength of the magnetic field, the number of available diffusion gradient directions, the choice of b-value(s), and the spatial resolution – all of which may have impacted the sensitivity of the analyses. These issues clearly need to be addressed systematically in future studies. Indeed, guidelines have recently been published with the goal of harmonizing imaging studies in cancer populations [Citation53].
The hippocampal regions were one of few brain structures to be investigated specifically. Of six studies, four indicated alterations in this region [Citation27,Citation33,Citation34,Citation36]. All of these studies, however, were cross-sectional and mainly compared patients with HC. It is worth noting that the largest study to date with long-term BC survivors did not find signs of hippocampal volume reductions [Citation24]. Also, the only study to contrast CT + with CT − failed to detect any differences [Citation32].
Only four publications applied network analysis [Citation23,Citation37,Citation39,Citation42]. However, network impairments were reported in all of these, including one that compared BC patients prior to treatment with HC [Citation37]. The only longitudinal study to date, reported significant changes in central network parameters in CT + compared with CT− [Citation42]. Network analysis of structural imaging data is a relatively novel approach to assess the overall topological organization of brain networks. Because this approach is inherently multivariate, it may be more sensitive to detecting subtle brain alterations.
In terms of study design, heterogeneity in time since treatment, cancer type, imaging methods used, and anatomical regions examined, prevented meaningful comparisons of longitudinal versus cross-sectional findings. For example, in BC, most of the longitudinal studies assessed brain changes from pretreatment to shortly after treatment completion (usually within a year), whereas cross-sectional studies were often conducted several years after treatment completion. In the case where a cross-sectional study was comparable to two longitudinal studies in terms of time since treatment, imaging modality, and structures examined, cross-sectional findings corroborated longitudinal findings [Citation21,Citation28,Citation35].
When associations between structural brain properties and objective cognitive outcomes were examined, half of those studies reported significant associations. Although the expected cognitive domains of processing speed, attention/working memory, and memory were detected across a number of those studies, they were associated with heterogeneous regions and properties, and were not the only cognitive domains associated with structural regions. The remaining studies detected no significant correlations or did not test for associations. In short, there was no clear association between specific structural properties or regions with specific cognitive domains, and where associations existed, they were highly distributed – consistent with findings in healthy populations [Citation54]. A new conceptualization of the connection between brain structure and cognitive functioning is likely to be necessary – potentially one that engages network science in both structural and functional imaging to better illuminate dynamic human cognitive architectures [Citation37].
In addition to the aforementioned limitations, additional limitations may limit the interpretation of findings. One limitation is that the extent of structural impairments may have been related to the choice of comparison group. The most consistent differences were observed between CT + and HCs, which do not directly elucidate treatment-specific effects. Generally, when comparisons were made between a specific treatment group and a treatment-naïve group, structural alterations were more subtle. In addition, most of the studies were restricted by small sample sizes and high between-study heterogeneity in important imaging and analytical variables. Finally, the effect of hormones or menopausal status may affect the brain [Citation33], but most studies mixed pre- and postmenopausal patients and individuals on anti-estrogen therapies.
In sum, there is both cross-sectional and longitudinal evidence to indicate that structural brain alterations may follow cancer and its treatment. Neuroimaging has clearly become an important research methodology within CRCI as it allows for non-invasive investigations of neurobiological underpinnings. However, given recent replicability issues in neuroimaging research, it is imperative that future large-scale studies replicate and build upon initial findings. Moreover, given recent developments in hormonal and immune therapies that may last from several years to end of life, longitudinal studies with long-term follow-ups are warranted. A greater focus on the role of moderators such as specific risk polymorphisms, cognitive reserve, age, and the effect of time since treatment also need to be examined. Finally, preregistration of studies is recommended to mitigate the potential risk of selective reporting [Citation55].
The present review has several limitations. First, neuroimaging was restricted to structural imaging, although a number of studies examined both structure and function. It would likely be fruitful to combine findings from both imaging approaches to better understand the dynamic interplay between structure and function in the context of CRCI. Second, due to differences in structural imaging outcomes, as well as the particular format of neuroimaging results, meta-analysis was not possible. Third, the quality assessment tool used in this review, although useful for appraising general risk of bias, was not specifically geared towards imaging studies. Finally, a specific focus on moderating risk factors of brain structural alterations was outside the scope of this review.
Supplemental Material
Download Zip (39.5 KB)Disclosure statement
The authors declare no conflict of interests. This work was supported by the Danish Council of Independent Research under Grant DFF – 5053-00220B; the Danish Cancer Society under Grant R174-A11447-17-S52 (Dr. Amidi); and the National Cancer Institute of the National Institutes of Health under Award Number 5K07CA184145-03 (Dr. Wu). Content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Additional information
Funding
References
- Bernstein LJ, McCreath GA, Komeylian Z, et al. Cognitive impairment in breast cancer survivors treated with chemotherapy depends on control group type and cognitive domains assessed: a multilevel meta-analysis. Neurosci Biobehav Rev. 2017;83:417–428.
- Lange M, Giffard B, Noal S, et al. Baseline cognitive functions among elderly patients with localised breast cancer. Eur J Cancer. 2014;50:2181–2189.
- Cleeland CS, Bennett GJ, Dantzer R, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–2925.
- Irwin M. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66:143–172.
- Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172:1075–1091.
- Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications [Internet]. Pharmacol Ther. 2011;130:226–238.
- Innominato PF, Roche VP, Palesh OG, et al. The circadian timing system in clinical oncology. Ann Med. 2014;46:191–207.
- Mandelblatt JS, Hurria A, McDonald BC, et al. Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know? Semin Oncol. 2013;40:709–725.
- Lomeli N, Di K, Czerniawski J, et al. Cisplatin-induced mitochondrial dysfunction is associated with impaired cognitive function in rats. Free Radic Biol Med. 2017;102:274–286.
- Wang XM, Walitt B, Saligan L, et al. Chemobrain: a critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy [Internet]. Cytokine. 2015;72:86–96.
- Castel H, Denouel A, Lange M, et al. Biomarkers associated with cognitive impairment in treated cancer patients: potential predisposition and risk factors. Front Pharmacol. 2017;8:138.
- Ashburner J, Friston KJ. Voxel-based morphometry – the methods. Neuroimage. 2000;11:805–821.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
- Stouten-Kemperman MM, de Ruiter MB, Koppelmans V, et al. Neurotoxicity in breast cancer survivors ≥10 years post-treatment is dependent on treatment type. Brain Imaging Behav. 2015;9:275–284.
- Billiet T, Emsell L, Vandenbulcke M, et al. Recovery from chemotherapy-induced white matter changes in young breast cancer survivors? Brain Imaging Behav. 2018;12:64–77.
- Sato C, Sekiguchi A, Kawai M, et al. Postoperative structural brain changes and cognitive dysfunction in patients with breast cancer. Chen K, editor. PLoS One. 2015;10:e0140655.
- Koppelmans V, de Groot M, de Ruiter MB, et al. Global and focal white matter integrity in breast cancer survivors 20 years after adjuvant chemotherapy. Hum Brain Mapp. 2014;35:889–899.
- Lepage C, Smith AM, Moreau J, et al. A prospective study of grey matter and cognitive function alterations in chemotherapy-treated breast cancer patients. Springerplus. 2014;3:444.
- Conroy SK, McDonald BC, Smith DJ, et al. Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res Treat. 2013;7(4):493–502.
- McDonald BC, Conroy SK, Smith DJ, et al. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain Behav Immun. 2013;30:S117–S125.
- Deprez S, Amant F, Smeets A, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. JCO. 2012;30:274–281.
- De Ruiter MB, Reneman L, Boogerd W, et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Mapp. 2012;33:2971–2983.
- Hosseini SMH, Koovakkattu D, Kesler SR. Altered small-world properties of gray matter networks in breast cancer. BMC Neurol. 2012;12:28.
- Koppelmans V, de Ruiter MB, van der Lijn F, et al. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat. 2012;132:1099–1106.
- Scherling C, Collins B, MacKenzie J, et al. Structural brain differences in breast cancer patients compared to matched controls prior to chemotherapy. Int J Biol. 2012;4:p3.
- Chen BT, Sethi SK, Jin T, et al. Assessing brain volume changes in older women with breast cancer receiving adjuvant chemotherapy: a brain magnetic resonance imaging pilot study. Breast Cancer Res. 2018;20:38.
- Bergouignan L, Lefranc JP, Chupin M, et al. Breast cancer affects both the hippocampus volume and the episodic autobiographical memory retrieval. PLoS One. 2011;6:e25349.
- Deprez S, Amant F, Yigit R, et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp. 2011;32:480–493.
- McDonald BC, Conroy SK, Ahles TA, et al. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat. 2010;123:819–828.
- Inagaki M, Yoshikawa E, Matsuoka Y, et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109:146–156.
- Abraham J, Haut MW, Moran MT, et al. Adjuvant chemotherapy for breast cancer: effects on cerebral white matter seen in diffusion tensor imaging. Clin Breast Cancer. 2008;8:88–91.
- Yoshikawa E, Matsuoka Y, Inagaki M, et al. No adverse effects of adjuvant chemotherapy on hippocampal volume in Japanese breast cancer survivors. Breast Cancer Res Treat. 2005;92:81–84.
- Eberling JL, Wu C, Tong-Turnbeaugh R, et al. Estrogen- and tamoxifen-associated effects on brain structure and function. Neuroimage. 2004;21:364–371.
- Kesler S, Janelsins M, Koovakkattu D, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013;30:S109–S116.
- Menning S, de Ruiter MB, Veltman DJ, et al. Changes in brain white matter integrity after systemic treatment for breast cancer: a prospective longitudinal study. Brain Imaging Behav. 2018;12:324–334.
- Apple AC, Ryals AJ, Alpert KI, et al. Subtle hippocampal deformities in breast cancer survivors with reduced episodic memory and self-reported cognitive concerns. NeuroImage Clin. 2017;14:685–691.
- Kesler SR, Adams M, Packer M, et al. Disrupted brain network functional dynamics and hyper-correlation of structural and functional connectome topology in patients with breast cancer prior to treatment. Brain Behav. 2017;7:e00643.
- Mo C, Lin H, Fu F, et al. Chemotherapy-induced changes of cerebral activity in resting-state functional magnetic resonance imaging and cerebral white matter in diffusion tensor imaging. Oncotarget. 2017;8:81273–81284.
- Kesler SR, Watson CL, Blayney DW. Brain network alterations and vulnerability to simulated neurodegeneration in breast cancer. Neurobiol Aging. 2015;36:2429–2442.
- Koppelmans V, Vernooij MW, Boogerd W, et al. Prevalence of cerebral small-vessel disease in long-term breast cancer survivors exposed to both adjuvant radiotherapy and chemotherapy. JCO. 2015;33:588–593.
- Menning S, de Ruiter MB, Veltman DJ, et al. Multimodal MRI and cognitive function in patients with breast cancer prior to adjuvant treatment—the role of fatigue. NeuroImage Clin. 2015;7:547–554.
- Amidi A, Hosseini SMH, Leemans A, et al. Changes in brain structural networks and cognitive functions in testicular cancer patients receiving cisplatin-based chemotherapy. J Natl Cancer Inst. 2017;109:9041–9044.
- Stouten-Kemperman MM, de Ruiter MB, Caan MWA, et al. Lower cognitive performance and white matter changes in testicular cancer survivors 10 years after chemotherapy. Hum Brain Mapp. 2015;36:4638–4647.
- Amidi A, Agerbaek M, Wu LM, et al. Changes in cognitive functions and cerebral grey matter and their associations with inflammatory markers, endocrine markers, and APOE genotypes in testicular cancer patients undergoing treatment. Brain Imaging Behav. 2017;11:769–783.
- Correa DD, Wang Y, West JD, et al. Prospective assessment of white matter integrity in adult stem cell transplant recipients. Brain Imaging Behav. 2016;10:486–496.
- Correa DD, Root JC, Baser R, et al. A prospective evaluation of changes in brain structure and cognitive functions in adult stem cell transplant recipients. Brain Imaging Behav. 2013;7:478–490.
- Simó M, Root JC, Vaquero L, et al. Cognitive and brain structural changes in a lung cancer population. J Thorac Oncol. 2015;10:38–45.
- Chao HH, Hu S, Ide JS, et al. Effects of androgen deprivation on cerebral morphometry in prostate cancer patients – an exploratory study. Wilson E, editor. PLoS One. 2013;8:e72032.
- Correa DD, Root JC, Kryza-Lacombe M, et al. Brain structure and function in patients with ovarian cancer treated with first-line chemotherapy: a pilot study. Brain Imaging Behav. 2017;11:1652–1663.
- Hatzimichael E, Tuthill M. Hematopoietic stem cell transplantation. Stem Cells Cloning Adv Appl. 2010; 3:105–117.
- Hsu JL, Chen YL, Leu JG, et al. Microstructural white matter abnormalities in type 2 diabetes mellitus: a diffusion tensor imaging study. Neuroimage. 2012;59:1098–1105.
- Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329.
- Deprez S, Kesler SR, Saykin AJ, et al. International cognition and cancer task force recommendations for neuroimaging methods in the study of cognitive impairment in non-CNS cancer patients. J Natl Cancer Inst. 2018;110:223–231.
- Takeuchi H, Taki Y, Nouchi R, et al. Global associations between regional gray matter volume and diverse complex cognitive functions: evidence from a large sample study. Sci Rep. 2017;7:10014.
- General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81:14–18.