Abstract
Background: Glioblastoma (GBM) is an aggressive brain tumor with a short overall survival (OS) in general. The treatment of GBM has evolved over the last decades and is today multimodal including surgical resection followed by radiochemotherapy and adjuvant chemotherapy for patients in good performance status. The aim of this study was to evaluate the development of treatment and the outcome for GBM patients at a single regional center.
Patients and methods: Survival was studied for 571 patients in our region diagnosed with GBM between 1995 and 2015. Samples from 244 patients out of those treated 2005–2015 have been included in a tissue/blood bank and a clinical database has been set up with basic patient characteristics and details on surgery and non-surgical treatment.
Results: The median OS for all patients from 1995 to 2015 was 9.3 months. There was a stepwise improvement from 6.9 to 10.3 months for patients diagnosed 1995–1996 and 2010–2015, respectively (p < .05). The 2-year survival for the same time periods improved from 7% to 18% (p < .01). After introduction of postoperative radiochemotherapy for patients in good performance status in 2005 an increased OS was noted and following implementation of intraoperative 5-aminolevulinic acid the number of tumor resection ≥95% did increase from 33% to 54% (p < .001). Positive prognostic factors for survival were young age, good performance status, absence of inflammatory disease, absence of diabetes or metabolic disease, tumor resection ≥95%, and completion of postoperative radiochemotherapy.
Discussion: The results of this study are consistent with earlier results regarding survival and prognostic factors and confirm results from randomized controlled trials in a clinical setting. Despite the improvements made, the prognosis is still dismal and the need for further research on GBM treatment is great.
Introduction
Glioblastoma (GBM) is the most common malignant primary brain tumor. In Sweden approximately 400 persons are diagnosed with GBM every year and the age-adjusted incidence in 2015 was 3.8 cases per 100,000 (data from the Swedish Cancer Registry). GBM is a rapidly progressing tumor characterized by an infiltrative growth pattern making local treatments such as surgery or radiotherapy insufficient [Citation1]. Despite modern state-of-the-art treatment median OS is still a disappointing 10–12 months outside clinical trials [Citation2–Citation4].
Treatment of GBM has changed over the last 20 years. Surgically, the implementation of neuronavigation and the introduction of intraoperative 5-aminolevulinic acid (5-ALA) flourescent microsurgery have enhanced the possibilities to achieve a radiological gross total tumor resection [Citation5]. A higher extent of tumor resection is associated with improved survival [Citation3,Citation6–8]. Postoperative treatment with radiotherapy and temozolomide followed by adjuvant temozolomide has been introduced in clinical practice and is now standard of care for patients in good performance status [Citation9–11].
At tumor progression, there is no standard second line treatment established [Citation12–15]. The anti-vascular endothelial growth factor (VEGF) antibody bevacizumab has been used in a selected group of patients as monotherapy or in combination with chemotherapy. However, it is not approved in Europe for treatment of glioma and cannot be considered as standard treatment [Citation16–18]. At our center selected patients with progressive GBM have been offered bevacizumab-based second line treatment since 2007 [Citation19].
Much of the reported improvements in survival have been results from clinical trials in selected groups of patients. Reports on population-based results over longer time periods are sparse but important since it more accurate reflects the group of patients that we meet on a daily basis [Citation3,Citation4,Citation20,Citation21]. The aim of the present study was to evaluate how the scientific progress in GBM treatment has been translated into clinical reality for the whole population of patients treated at a single Swedish university hospital from 1995 to 2015 and to what extent it may have influenced survival. For a subgroup of patients, included in the biobank cohort 2005–2015, we have collected clinical data in order to describe the cohort in detail. Inclusion in the biobank is prospective and patients included in the biobank cohort after 2010 is participating in the Uppsala-Umeå comprehensive cancer consortium (U-CAN), which is a joint project with Uppsala University [Citation22].
Material and methods
Patients
Umeå University Hospital is serving the northern Swedish health care region (NSHCR) with a population of 920,357–884,384 during the years of the study. GBM care is largely centralized to the university hospital where all patients are monitored at the multidisciplinary brain tumor board for treatment decisions. Through the Swedish Cancer Registry, the medical records from the departments of Neurosurgery and Oncology, Umeå University Hospital, and the database of the biobank, adult patients with a histopathological diagnosis of intracranial GBM according to the WHO classification during the years 1995–2015 in the NSHCR were identified. For all patients diagnosed 1995–2015 data regarding age, gender and survival was collected from the registry. Survival was, for all patients, calculated from the date of diagnosis, defined as the date of first surgical procedure. Patients that were alive at the end of the study, 3 September 2017, were censored.
In 2005 a biobank was set up and after 2010 this was also part of the U-CAN project [Citation22]. In the biobank, tissue and blood samples are collected at surgery and thereafter blood samples at 3, 6, and 12 months. Up to 2015, 244 patients with GBM were included in the biobank cohort representing 64% of all 382 patients diagnosed in NSHCR during these years. For patients contributing to the biobank, clinical information regarding age, gender, performance status according to WHO [Citation23], comorbidity, postoperative radiotherapy and/or chemotherapy, corticosteroid use, and treatment at tumor progression were retrospectively collected from the medical records (NCS Cross® (EVRY, Stockholm, Sweden), VAS® (Norrbottens läns landsting, Luleå, Sweden), and Cosmiq® (Cambio Healthcare Systems, Stockholm, Sweden). Comparisons between the patients included in the biobank cohort and the patients diagnosed during the same years, 2005–2015, that were not included in the biobank cohort were performed.
Radiologic evaluation
Volumetric measurements of pre- and postoperative tumor volume were performed in the SECTRA® (Sectra, Linköping, Sweden) system on T1-weighted magnetic resonance imaging (MRI) with gadolinium contrast. Using pixel spacing, the contrast-enhancing lesion was outlined on axial images, producing a cross-section area (mm2) of the tumor in the given slice. The area was multiplied with slice thickness to calculate tumor volume (mm3) in each slice. Tumor volume in each slice was then added to obtain total tumor volume. Measurements were performed on computed tomography (CT)-scans when there was no preoperative MRI available or when the MRI was performed >3 weeks prior to surgery and a more recent CT was available. When there was no postoperative MRI, measurement was done on CT-scans.
Tumor location was defined as frontal, parietal, temporal, occipital, central, multifocal, or posterior fossa. The following areas were considered eloquent: motor or sensory cortex, visual cortex, speech areas, internal capsule, basal ganglia, hypothalamus or thalamus, brainstem, and dentate nucleus.
Treatment
In order to analyze the treatment results over time, patients were divided into four groups according to year of diagnosis. The groups were the following: 1995–1996, 1997–2004, 2005–June 2010 (Q2 2010), and July 2010 (Q3 2010) –2015. The cutoff points were chosen considering three important changes in diagnostics and treatment: a revised WHO classification [Citation24] was implemented in 1997, radiochemotherapy according to the trial by EORTC/National Cancer Institute of Canada Clinical Trials Group [Citation9] was initiated in 2005 and the use of 5-ALA at surgery was started in July 2010.
Surgical treatment was graded as biopsy, resection <95% or resection ≥95% based on the volumetric measurements. In seven patients, where neither postoperative MRI nor CT was performed, the postoperative tumor volume was estimated as partial or total resection, based on the surgeon’s judgment. For patients diagnosed 2005–2015 not included in the biobank cohort, the degree of resection was obtained from the surgical notes reporting the surgeon’s subjective assessment.
Radiochemotherapy was defined as radiotherapy to 60 Gray (Gy) in 30 fractions with concomitant temozolomide followed by six cycles of adjuvant temozolomide [Citation9]. Radiotherapy was delivered as 3D conformal radiotherapy, intensity modulated radiotherapy (IMRT) or volumetric arc therapy (VMAT) using high-energy linear accelerators.
In the analysis of non-surgical treatment, patients were divided into four subgroups according to given postoperative treatment and a fifth group who did not receive any radio- or chemotherapy. Groups were defined as follows: patients who received completed postoperative radiochemotherapy (including patients treated with 6 or more cycles of adjuvant temozolomide; reduced temozolomide dose or delayed administration due to hematologic toxicity was accepted); not completed postoperative radiochemotherapy; other treatment (e.g., radiotherapy or temozolomide as monotherapy, hypofractionated radiotherapy followed by adjuvant temozolomide, temozolomide as monotherapy before initiation of radiochemotherapy or treatment in clinical studies including radiotherapy and/or temozolomide); and patients included in postoperative clinical studies with bevacizumab [Citation25,Citation26] or valganciclovir in combination with radiotherapy and temozolomide [Citation27]. All patients scheduled for radiochemotherapy constitute the intention to treat (ITT) radiochemotherapy group.
Repeated surgery, systemic therapy and radiotherapy at tumor progression were registered. Patients included in clinical studies at tumor progression were classified according to their primary treatment.
MGMT analysis
In a subgroup of the biobank cohort, MGMT promoter methylation was analyzed in tissue from 85 patients in the ITT radiochemotherapy group.
DNA extraction and bisulfite treatment
Bisulfite conversion of 50 ng genomic DNA was performed using the EpiTect bisulfite kit (ref. 59124, Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Each bisulfite modification experiment included a universal 50% methylated DNA sample prepared by mixing a 100% methylated sample and an unmethylated sample (Human Methylated & Nonmethylated DNA Set, catalog code: D5014, Zymo Research). DNA from a normal brain as negative control was included.
Analysis of MGMT promoter methylation
The pyrosequencing assay was performed using the therascreen MGMT Pyro Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The PCR products were subjected to pyresequencing on a Pyromark Q24 System (Qiaqen, Hilden, Germany).
Statistical analyses
A database was set up using MS Excel (Microsoft Corp, Redmond, WA) and statistical analyses were performed using SPSS 24 (IBM Corp, Armonk, NY). When comparing the two cohorts comparisons were done with Chi-square test and Student’s t-test. Pearson Chi-square test was used to determine if there was any correlation between gender, age group and type of surgery and also if there was any correlation between comorbidity or smoking and survival. Univariate survival analysis was performed using Kaplan–Meier estimates and subgroups were compared with log-rank test. Multivariable survival analysis was performed using Cox regression. Results were considered significant when p < .05.
Ethics
Ethical approval of this study was obtained from the Ethics Committee at Umeå University (2003/03-154, 2006/06/124, 2011-308-31M, 2016-95-32M, 2016-188-32M).
Results
Patients
During the years 1995–2015, 571 adult patients diagnosed with GBM in the NSHCR were identified (). Twenty patients (4%) were censored at a median follow up time of 48.7 months (range 21.1–153.2 months). For all 571 patients the median age at diagnosis was 64 years (range 19–84), 352 were male (62%) and 219 female (38%). In 1993, there was a change in the WHO classification that was implemented on a national level in 1997. This resulted in an increase in the incidence of GBM and decrease in, especially, anaplastic astrocytoma WHO grade III ( and Supplementary Figure 1).
Table 1. Incidence, basic characteristics and overall survival of 571 adult patients with GBM in northern Sweden during the years 1995–2015.
During the years 2005–2015, 382 patients were identified (Supplementary Table 1) of whom 244 patients (64%) contributed to the biobank. One hundred and thirty patients (34%) not included in the biobank cohort and eight patients (2%) diagnosed by autopsy in the same time period were also identified.
In the biobank cohort, 155 were male (64%) and 89 female (36%) and the median age was 64 years (range 21–84). Cardiovascular disease and metabolic disease including diabetes were common. Preoperative WHO performance status was 0–1 for a majority of the patients (Supplementary Table 1). The basic characteristics of the 138 patients diagnosed 2005–2015, but not included in the biobank cohort, are also presented in Supplementary Table 1.
Survival
For all 571 patients, the median OS was 9.3 months (95% CI 8.5–10.1). The median OS improved from 6.9 months (95% CI 5.3–8.6) for patients diagnosed 1995–1996 to 10.3 months (95% CI 8.9–11.7) for patients diagnosed after July 2010 ( and ). A stepwise increase was noted especially in 2- and 3-year survival (). For the biobank cohort the median OS was 10.6 months (95% CI 9.6–11.7). The 138 patients not included in the biobank cohort had a significantly shorter median OS, 6.8 months (95% CI 5.6–8.0, p < .05) (Supplementary Table 1).
Figure 1. Kaplan–Meier analysis of overall survival in 571 patients with GBM diagnosed from 1995 to 2015. All patients (a), by year of diagnosis (b). Number at risk is presented below the Kaplan–Meier plot. Log-rank test.
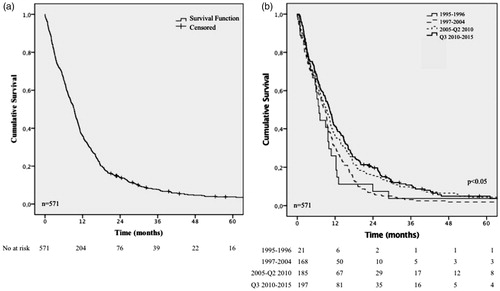
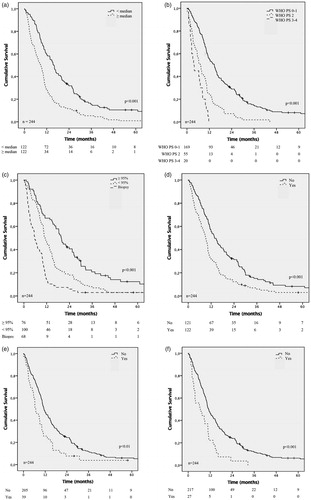
Figure 2. Kaplan–Meier analysis of overall survival in 244 patients diagnosed with GBM during the years 2005–2015 (the biobank cohort). Effect on overall survival of patient age (a), preoperative performance status (b), type of surgery (c), cardiovascular disease (one missing) (d), diabetes/metabolic disease (e), and inflammatory disease (f). Number at risk is presented below the Kaplan–Meier plot. Log-rank test.
Treatment and survival in the biobank cohort
Surgery
In the biobank cohort, tumor resection ≥95% was achieved in 76 patients (31%), 100 patients (41%) underwent a tumor resection <95%, and 68 patients (28%) had a tumor biopsy. Forty-nine patients (20%) had tumors located in an eloquent area (Supplementary Table 2).
5-ALA was introduced in July 2010 and was thereafter used in 85 (79%) of all tumor resections. When using 5-ALA, the number of patients that underwent a resection ≥95%, was significantly higher (54% versus 33%, p < .001). Tumor resection ≥95% is not always possible due to tumor location but, in our study, the number of resections ≥95% in eloquent areas (41%) was similar to non-eloquent areas (44%) for patients with tumor resection. For patients with tumor resections, the mean preoperative tumor volume was 37.9 cm3 and the mean postoperative tumor volume was 4.1 cm3 (p < .001) (Supplementary Table 2).
The postoperative WHO performance status was improved for one-third, worse for one-third of the patients, and for the remaining patients no change was registered compared to preoperative performance status (data not shown).
Non-surgical treatment
Radiochemotherapy was introduced in 2005 and was thereafter used in 134 patients (55%) and 42 (31%) out of those completed the treatment (Supplementary Table 3). For patients initiating radiochemotherapy a mean of 3.5 cycles of adjuvant temozolomide was administered. Tumor progression was the most common cause for interrupted treatment and toxicity, mostly hematologic, was the second most common cause (Supplementary Table 4). In the ITT group, there was no difference in gender distribution, age or use of corticosteroids during radiotherapy between patients that completed or interrupted their therapy. For the patients that interrupted radiochemotherapy, performance status was worse (p < .05) (Supplementary Table 4).
Nineteen patients (8%) were included in clinical trials with addition of experimental drugs to postoperative radiochemotherapy and 68 patients (28%) underwent postoperative treatment differing from that previously described including radiotherapy (n = 28) or chemotherapy (n = 18) as monotherapy and other postoperative treatment (n = 22) (Supplementary Table 3).
Treatment at tumor progression
One hundred and twenty-six (52%) patients had antineoplastic treatment at tumor progression; 50 patients (20%) had repeated surgery, 66 patients (27%) had chemotherapy and/or bevacizumab, and 10 patients (4%) underwent radiotherapy at tumor progression. Repeated surgery was combined with systemic therapy in 44 patients (18%) (Supplementary Figure 2 and Supplementary Table 5). Three patients were still under observation without sign of tumor progression up until September 2017.
Detailed analyses of survival in the biobank cohort
Patients with a tumor resection ≥95% had a median OS of 19.6 months (95% CI 17.4–21.9), patients that underwent a resection <95% had a median OS of 11.0 months (95% CI 9.1–12.9) and for biopsy the median OS was significantly shorter, 5.7 months (95% CI 4.2–7.2) ( and Supplementary Table 2).
The median OS for the ITT radiochemotherapy group was 15.0 months (95% CI 13.0–17.2). Further, the 2-year-survival was 29% and the 5-year survival 3% (Supplementary Table 4). For patients with antineoplastic treatment at tumor progression, the median OS was 18.8 months (95% CI 16.1–21.5) (Supplementary Table 5).
MGMT promoter methylation was analyzed in 85 patients (63%) in the ITT radiochemotherapy population. The 15 patients with high promoter methylation (>25%) had a median OS of 38.2 months (95% CI 12.0–46.1), nine patients with low MGMT promoter methylation (10–25%) had a median OS of 18.5 months (95% CI 6.9–11.2) and 61 patients with unmethylated MGMT promoter (<10%) had a median OS of 19.1 months (95% CI 13.4–18.0) (Supplementary Figure 3).
The use of corticosteroids during radiochemotherapy was associated with a shorter OS. In the ITT radiochemotherapy group, 97% of the patients were given corticosteroids after a tumor biopsy, 72% of the patients had corticosteroids after a tumor resection <95%, and 44% after a tumor resection ≥95%. The group treated with corticosteroids during radiotherapy had a significantly larger mean postoperative tumor volume (10.6 cm3 compared to 1.6 cm3 (p < .001)) (data not shown).
Cox regression analyses revealed that age below 64 years, favorable preoperative WHO performance status, no diabetes/metabolic or inflammatory disease, extensive surgery and postoperative radiochemotherapy were independent factors associated with longer OS ( and ).
Table 2. Cox-regression analysis of factors associated with overall survival in 244 patients with GBM diagnosed 2005–2015 (the biobank cohort).
Discussion
During the last two decades, there has been a development in the treatment of GBM with improvements in surgical techniques and introduction of postoperative radiochemotherapy and adjuvant chemotherapy as standard treatment. In this study, the outcome of the treatment of GBM at the university hospital serving the northern part of Sweden is described. We found that stepwise changes in treatment routines have translated into an improved outcome for our patients, especially in 2-year survival, and we have been able to identify subgroups with substantially better prognosis.
Analyzing our survival data, we found three factors that seemed to influence the overall survival. First, the revised WHO classification in 1993 that, when implemented in 1997, resulted in an increase in the incidence of GBM and decrease of, especially, astrocytoma III (Supplementary Figure 1) [Citation24]. Most probably, this had the effect that some tumors earlier classified as grade III was classified as grade IV tumors resulting in a longer median survival for the group of grade IV tumors diagnosed after 1997. This diagnostic drift due to the change of the WHO classification of CNS tumors may be of great importance when comparing modern studies with historical data.
Second, in 2005, postoperative radiochemotherapy was introduced in clinical routine [Citation9] and we found an OS comparable to the 5-year analysis of the EORTC-NCIC trial [Citation10] regarding 2-year survival (29% in our cohort compared to 27% in the EORTC-NCIC trial) for patients initiating combined treatment. Despite that the patients in our cohort are older and had a worse performance status. The 5-year survival was lower in our study compared to the EORTC-NCIC trial, 3% and 10%, respectively [Citation9,Citation10]. These findings implicates that the results of the EORTC-NCIC trial are reproduced in a population based clinical cohort. As expected, patients with MGMT promoter methylated tumors had a similar survival benefit as been reported in many previous studies [Citation28,Citation29].
Third, after July 2010 the use of 5-ALA became routine during surgery [Citation5] which, in patients that underwent resection, resulted in a significantly higher number of patients operated on with tumor resection ≥95%, figures coinciding with previous studies on 5-ALA [Citation5]. In our study, as well as in many others, extensive surgery was independently associated with a longer OS [Citation7,Citation30]. These data must be considered with caution since those patients are not subject to randomization. Although more patients had resections ≥95% after the introduction of 5-ALA, the changes in postoperative performance status in our series was comparable to other studies [Citation20,Citation31].
The survival of our unselected patient cohort over time is in line with earlier findings [Citation2,Citation3,Citation21]. However, in a study from Kawano et al. [Citation32], the OS was slightly longer. One explanation could be that more patients in our study had undergone biopsy only, which may indicate a possible selection bias in the Japanese study. There were also differences in postoperative treatment, both regarding radiotherapy and chemotherapy between the studies that partly may explain the different outcomes. The prognostic factors that we found to be associated with a survival benefit are in concordance with earlier studies: younger age, favorable performance status, extensive surgery, and multimodal postoperative treatment [Citation3,Citation32,Citation33].
The subgroup of patients treated with extensive surgery and postoperative radiochemotherapy had a better OS. Unfortunately, only a minority of the patients completed radiochemotherapy and the long-term survivors in our cohort are few. The most frequent reason for abrogated postoperative treatment in our series was tumor progression. In the EORTC-NCIC study by Stupp et al. [Citation9] a median of three cycles of adjuvant temozolomide was administered and 47% of the patients completed six cycles. The main reason for abrogated treatment in their study was disease progression and only in 8% the reason was toxicity. In comparison, toxicity from treatment was more common in our study (25%), which may be related to that our patients had a worse performance status and a higher median age. Misinterpretation of pseudoprogression as true progression could also be a reason for abrogated adjuvant chemotherapy in some cases. In order to minimize this problem, our patients are always discussed at the multidisciplinary brain tumor board to consider both clinical status and radiology findings to determine if to continue or stop/change treatment.
Patients with a tumor resection ≥95% followed by completed radiochemotherapy had an outstanding prognosis with a median OS of 32.4 months. Of these 20 patients, 17 had antineoplastic treatment at tumor progression and three are still under observation without sign of progression. Whether the strong positive prognosis in this group depends on the treatment itself and/or that the patients belong to a group with strong prognostic clinical factors is in the absence of a randomized controlled trial, impossible to know. Future studies on genetics, proteomics, or metabolomics of these patients’ tumors may reveal clinically useful biomarkers.
Evidence for the value of treatment at tumor progression is limited. Our policy has been to, if the patient is in a good performance status, reoperate if possible and give second line non-surgical treatment. Temozolomide has been considered since 2005 if not administered earlier or if more than six months has passed since earlier temozolomide treatment. Further treatment (bevacizumab, irinotecan, lomustine, or a combination of procarbacine, lomustine, and vincristine) has been offered for patients in good performance status.
Limitations
When collecting data on performance status retrospectively from medical records, there is always a possibility for incorrect classification. In case of indistinct records, two of the authors did independently classify the patient after which a consensus was achieved.
MGMT status was only available for a subgroup of our ITT radiochemotherapy population which is a limitation in our study. The positive prognostic value of MGMT methylation is well recognized in many previous papers [Citation29,Citation34,Citation35].
The in-detail studied biobank cohort is not fully representative for the total patient cohort. Patients not included in the biobank cohort were usually older and more often had a biopsy as only surgical intervention. The amount of tissue from a biopsy was often too small to allow both histopathological diagnosis and a sample for the biobank. Since this group is not thoroughly studied, differences in postoperative treatment are not known. The shorter survival in this group can be related to older age and higher incidence of biopsies.
Conclusions
Our study demonstrates that improved GBM treatment with introduction of a multimodal approach increased survival in an unselected clinical cohort. This was especially evident in the improved 2-year survival rate from 7% 1995–1996 to 18% 2010–2015. However, the increase in median OS was modest (6.9–10.3 months), which emphasizes the need for further improvement in the treatment of GBM. New treatment strategies must be developed and tested in randomized controlled trials.
Based on the findings from this retrospective analysis, we would like to emphasize the value of multimodal treatment for patients in good performance status. Treatment at relapse including repeated surgery and second-line systemic treatment appears to be justified for a selected group of patients.
| Abbreviations | ||
| 5-ALA | = | 5-aminolevulinic acid |
| CT | = | computed tomography |
| GBM | = | glioblastoma |
| Gy | = | Gray |
| MGMT | = | 06-methylguanine-DNA-methyltransferase |
| MRI | = | magnetic resonance imaging |
| NSHCR | = | the northern Swedish health care region |
| VEGF | = | vascular endothelial growth factor |
Supplemental Material
Download Zip (247.8 KB)Acknowledgments
The authors are thankful to Thomas Lindqvist who supervised the radiological evaluation. Kristin Nyman, Mikael Kimdal, and Kristina Lundqvist are acknowledged for excellent technical support in collecting data to the study. The authors want to acknowledge all the patients and their families, contributing to the biobank cohort.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710.
- Asklund T, Malmström A, Bergqvist M, et al. Brain tumors in Sweden: data from a population-based registry 1999–2012. Acta Oncol. 2015;54:377–384.
- Laws ER, Parney IF, Huang W, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99:467–473.
- Gramatzki D, Dehler S, Rushing EJ, et al. Glioblastoma in the Canton of Zurich, Switzerland revisited: 2005 to 2009. Cancer. 2016;122:2206–2215.
- Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401.
- Tsitlakidis A, Foroglou N, Venetis CA, et al. Biopsy versus resection in the management of malignant gliomas: a systematic review and meta-analysis. J Neurosurg. 2010;112:1020–1032.
- Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014;16:113–122.
- Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–766.
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996.
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466.
- Perry JR, Laperriere N, O'Callaghan CJ, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376:1027–1037.
- Perry JR, Rizek P, Cashman R, et al. Temozolomide rechallenge in recurrent malignant glioma by using a continuous temozolomide schedule: the “rescue” approach. Cancer. 2008;113:2152–2157.
- Dresemann G. Imatinib and hydroxyurea in pretreated progressive glioblastoma multiforme: a patient series. Ann Oncol. 2005;16:1702–1708.
- Dresemann G, Weller M, Rosenthal MA, et al. Imatinib in combination with hydroxyurea versus hydroxyurea alone as oral therapy in patients with progressive pretreated glioblastoma resistant to standard dose temozolomide. J Neurooncol. 2010;96:393–402.
- Adair JE, Johnston SK, Mrugala MM, et al. Gene therapy enhances chemotherapy tolerance and efficacy in glioblastoma patients. J Clin Invest. 2014;124:4082–4092.
- Vredenburgh JJ, Desjardins A, Herndon JEII, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729.
- Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15:943–953.
- Erdem-Eraslan L, van den Bent MJ, Hoogstrate Y, et al. Identification of patients with recurrent glioblastoma who may benefit from combined bevacizumab and CCNU therapy: a report from the BELOB trial. Cancer Res. 2016;76:525–534.
- Sandström M, Laudius M, Lindqvist T, et al. A retrospective evaluation of bevacizumab treatment in patients with progressive malignant glioma in *Northern Sweden. Anticancer Res. 2017;37:1869–1874.
- Hansen S, Rasmussen BK, Laursen RJ, et al. Treatment and survival of glioblastoma patients in Denmark: the Danish Neuro-Oncology Registry 2009–2014. J Neurooncol. 2018;139:479–489.
- Zhu P, Du XL, Lu G, et al. Survival benefit of glioblastoma patients after FDA approval of temozolomide concomitant with radiation and bevacizumab: a population-based study. Oncotarget. 2017;8:44015–44031.
- Glimelius B, Melin B, Enblad G, et al. U-CAN: a prospective longitudinal collection of biomaterials and clinical information from adult cancer patients in Sweden. Acta Oncol. 2018;57:187–194.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655.
- Kleihues P, Burger P. Histological typing of tumours of the central nervous system. 2nd ed. Berlin: Springer; 1993.
- Chinot OL, Nishikawa R, Mason W, et al. Upfront bevacizumab may extend survival for glioblastoma patients who do not receive second-line therapy: an exploratory analysis of AVAglio. Neuro-oncology. 2016;18:1313–1318.
- Brandes AA, Ermani M, Basso U, et al. Temozolomide as a second-line systemic regimen in recurrent high-grade glioma: a phase II study. Ann Oncol. 2001;12:255–257.
- Stragliotto G, Rahbar A, Solberg NW, et al. Effects of valganciclovir as an add-on therapy in patients with cytomegalovirus-positive glioblastoma: a randomized, double-blind, hypothesis-generating study. Int J Cancer. 2013;133:1204–1213.
- Hegi ME, Liu L, Herman JG, et al. Correlation of O 6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. JCO. 2008;26:4189–4199.
- Hegi ME, Diserens A-C, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003.
- Bloch O, Han SJ, Cha S, et al. Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg. 2012;117:1032–1038.
- Chambless LB, Kistka HM, Parker SL, et al. The relative value of postoperative versus preoperative Karnofsky Performance Scale scores as a predictor of survival after surgical resection of glioblastoma multiforme. J Neurooncol. 2015;121:359–364.
- Kawano H, Hirano H, Yonezawa H, et al. Improvement in treatment results of glioblastoma over the last three decades and beneficial factors. Br J Neurosurg. 2015;29:206–212.
- Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198.
- Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354.
- Hegi ME, Diserens A-C, Godard S, et al. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res. 2004;10:1871–1874.
