Abstract
Background: Childhood cancer survivors treated before 1992, when blood donor screening for hepatitis C virus (HCV) infection was introduced, are at risk of transfusion-transmitted HCV infection. A national HCV screening campaign targeting blood transfusion recipients was launched in Sweden in 2007–2010. The aims of this study were to, among adult childhood cancer survivors in Stockholm County, investigate the prevalence of HCV infection, the natural course of infection, treatment outcome and anti-HCV testing frequency before, during and after the screening campaign and finally to actively screen the untested ones.
Material and Methods: This was a combined retrospective register based and prospective screening study of adult childhood cancer survivors (n = 686) treated for malignancy in Stockholm before 1992. In the first part, we investigated the prevalence of HCV infection and previous anti-HCV testing, and in the second part, we actively traced and HCV-screened the remaining untested cohort living in Stockholm. Analysis of previous documented anti-HCV tests in medical records, laboratory records, and the national communicable disease registry was performed. In the second part, 231 presumably untested individuals were contacted by mail and offered an anti-HCV test. The natural course of HCV infection and treatment outcome was analyzed for those found to be chronically infected.
Results: In total, 235 patients were tested and 11 were HCV-RNA positive. The overall prevalence of chronic HCV infection among the tested childhood cancer survivors was thus 4.7% (95% CI = 2.6–8.2%), which is almost 10 times higher than the national prevalence of 0.5%. Only 12% of the Stockholm cohort were tested during the screening campaign in 2007–2010, while the test uptake using active tracing screening within this study was 40% (p < .001).
Conclusion: With today’s effective treatment options, active tracing and HCV screening of childhood cancer survivors are recommended.
Background
Chronic hepatitis C virus (HCV) infection can lead to serious liver disease, and 20–30% of infected patients develop liver cirrhosis after 20–30 years’ duration [Citation1]. Chronic HCV infection is usually asymptomatic and only a minority of infected patients is aware of their status [Citation2]. The World Health Organization (WHO) has estimated that only 20% of all HCV infected patients are tested and diagnosed [Citation3]. In Sweden, blood transfusions were a common source of infection before blood donor screening for HCV was introduced in 1992 [Citation4]. During the mid-1980s the screening included so-called surrogate markers of blood-borne viral hepatitis (i.e., positive anti-Hepatitis B core and elevated alanine aminotransferase) as well as human immunodeficiency virus (HIV), which most likely excluded some but not all HCV positive blood donors [Citation5,Citation6].
Anemia is common during malignancy treatment and is often treated with blood transfusion. Transfusion rates in pediatric oncology treatment vary between 40% for brain tumors and 97% for leukemia [Citation7,Citation8]. Because of high transfusion rates, adult childhood cancer survivors treated before 1992 constitute a risk group of transfusion-transmitted HCV infection. Previous screening studies have shown a high HCV infection prevalence in this group of patients [Citation9,Citation10]. It has also been suggested that cancer survivors might develop a more aggressive HCV related liver disease due to the additional effect of cytotoxic and immunosuppressive treatment [Citation11].
This group of patients was never routinely screened for HCV infection in Sweden before, based on the conclusion that previous interferon-based HCV treatment was inefficient [Citation12,Citation13]. However, this decision was reevaluated later when new treatment options were developed, which led The Swedish National Board of Health and Welfare to launch a national hepatitis C screening campaign in 2007–2010 targeting blood transfusion recipients from 1965 to 1991 [Citation14]. The campaign information was given to the general population via posters, newspapers and websites. Childhood cancer survivors were one of three specifically targeted risk groups. However, analysis of the campaign outcome showed that only a small minority of the anti-HCV positive patients originated from the specific risk groups [Citation14,Citation15]. There are scarce data on the source of blood transfusion of the tested population but age analysis suggests that the campaign failed to reach the targeted pediatric risk groups since the majority of the tested population was older than expected [Citation15].
The aims of this study were to, among adult childhood cancer survivors treated in Stockholm County, investigate the prevalence of HCV infection, the natural course of infection, treatment outcome and also to determine how many had been tested for anti-HCV before, during and after the screening campaign and finally, to actively screen the untested individuals.
Patients and methods
This was a combined retrospective register based and prospective screening study of adult childhood cancer survivors treated for malignancy in Stockholm before 1992. In the first part of the study, prevalence of HCV infection and previous HCV testing were investigated and in the second part, the remaining untested cohort living in Stockholm County was screened for HCV.
Retrospective analysis of oncology register and medical records
We used a clinical pediatric oncology register of 2171 patients born 1913–1991 who had undergone investigation and/or treatment at the tertiary referral center of Pediatric oncology department at Karolinska University Hospital, Stockholm, Sweden from 1921 to 1991. Considering the relatively small number of patients treated during this long time period, we hypothesized that it only contained patients who survived treatment beyond a certain, but unknown, time point. The register did not contain information about previous blood transfusions and this was not possible to obtain since transfusion records from this time were not digitalized. Patients with benign diagnoses (n = 749), age above 18 years at initiation of therapy (n = 6) and patients registered without a complete personal identification number (n = 730) were excluded.
Patients in the included remaining cohort (n = 686) had been treated between 1952 and 1991. Analysis of existing notification of hepatitis C infection in the national register of chronic hepatitis C cases at the Public Health Agency was performed. To investigate the prevalence of previous HCV testing, medical records as well as laboratory registers were analyzed for documented anti-HCV tests. Only anti-HCV tests taken in Stockholm County were possible to track. Consequently, an unknown proportion of the patients may have been tested outside Stockholm. Medical record analysis was performed using the computerized hospital-based record system. Three different laboratory registers were analyzed for anti-HCV tests. All deceased patients were checked for liver-related cause of death and patients deceased before 1992 were excluded from further analysis.
Screening of untested patients in Stockholm county
In the second part of the study, the remaining group of patients without a previous traceable anti-HCV test who were still living in Stockholm County (n = 231) were contacted by letters and offered to participate in the HCV screening study. The informed consent had two alternatives: 1) those who already had tested negative for HCV, and 2) those still untested who opted to participate in screening. The latter group was offered a test for anti-HCV, free of charge at the nearest primary health care center. Two reminding letters were sent to those who did not reply. Patients were informed of test results by letters from the investigators. Analysis of age, gender, malignant diagnosis and time of treatment as well as results of anti-HCV screening were made.
The local ethics committee approved both parts of the study, including search for documented anti-HCV tests in medical records without informed consent from patients. Informed written consent was obtained from each patient for the active screening part of the study. The study was performed according to the World Medical Association Declaration of Helsinki.
Virological analyses
All anti-HCV analyses used in the active tracing screening were performed at the clinical routine laboratory at Karolinska University Hospital by third generation enzyme immuno assay (ARCHITECT Anti-HCV, Abbott, Wiesbaden, Germany). All HCV-RNA quantification analyses were performed at the clinical routine laboratory at Karolinska University Hospital by TaqMan real-time PCR (Roche Molecular Diagnostics, Branchburg, NJ, USA) with a detection limit of 15 IU/ml. HCV genotyping was performed with a line probe assay (Inno-LiPA HCV II, Innogenetics NV, Gent, Belgium).
Statistical analysis
Bivariate analysis of two dependent groups in evaluation of the effectiveness between the two screening methods was performed using McNemar's test. Statistical significance level was set at a two-sided p-value <.05. An exact 95% confidence interval for a proportion was calculated for the prevalence of HCV infection. Relative risk (RR) was calculated for the difference in national vs. cohort prevalence. The statistical analysis was performed using GraphPad Prism version 6.04 (GraphPad Prism Software, Inc., La Jolla, CA, USA).
Results
The included and excluded numbers of patients in the two parts of the study are shown in . Outcome and prevalence of testing are shown in Supplementary Figure S1.
Figure 1. All included and excluded patients in the study. Rectangles with sharp corners represent the first part of the study. Rectangles with rounded corners represent the second part of the study (the active tracing screening). HCV: Hepatitis C virus.
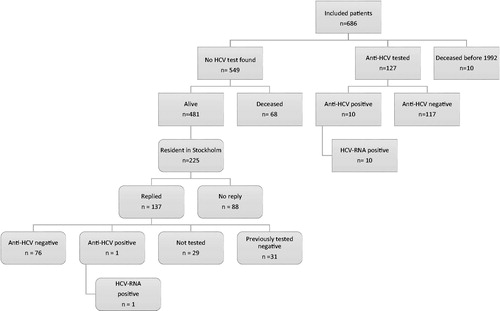
Results of the register and medical record analysis
Out of the total number of 2171 patients, 686 met the inclusion criteria. Malignancy diagnoses in this group were; solid tumors [n = 329 (48%)], leukemia [n = 157 (23%)], brain tumors [n = 91 (13%)], lymphoma [n = 74 (11%)], and other [n = 35 (5%)]. Ten died from malignancy related causes before 1992.
Out of 676 patients, 127 (19%, 60 women and 67 men) had a documented anti-HCV test taken in Stockholm before the year of 2016 (). Leukemia was the most common malignancy in the tested group (n = 51 (40%)). The remaining diagnoses were solid tumors (n = 41 (32%)), lymphomas (n = 16 (13%)), brain tumors (n = 9 (7%)), and other (n = 10 (8%)). Ten out of 127 tested patients were anti-HCV and HCV-RNA positive, diagnosing chronic HCV infection.
Table 1. Time point at first anti-HCV test in all tested patients.
Results of the active tracing screening in Stockholm in 2017–2018
Since 231 patients living in Stockholm did not have a traceable HCV test, an active tracing and HCV screening of this group was initiated in September 2017. They were treated for childhood malignancy between the years of 1965 and 1991. Six had recently moved outside Stockholm County and were excluded. Out of the remaining cohort of 225 patients, 137 replied and were included in the screening program, giving a response rate of 61% (137/225) (). The characteristics of all 225 patients in the actively traced and screened group are shown in . Out of the 137 patients, 31 had previously tested negative for anti-HCV (). The remaining 106 patients were not previously tested and accepted to be tested for anti-HCV ( and ). Despite two reminding letters, only 77 out of the 106 patients eventually were tested, giving a test frequency of 73% (). Patients treated for leukemia were more prone to participate (68%) and had a high testing frequency (84%), whereas patients treated for brain tumors were less prone to participate (49%) ().
Table 2. Characteristics of the actively screened cohort between the years 2017 and 2018.
In the actively traced group, 76 were anti-HCV negative and one tested positive for anti-HCV and HCV-RNA ().
Effectiveness of screening methods
For the Stockholm cohort of patients, a comparison of the effectiveness of the two screening methods was performed, that is, non-active tracing screening via public information posters (used in the screening 2007–2010) versus active tracing screening when patients were actively traced and contacted by mail (used in the screening 2017–2018). The non-active tracing screening reached 12% (37/303) of patients possible to screen of whom 5% (2/37) were HCV positive and the active tracing screening reached 40% (77/194) of whom 1% (1/77) were HCV positive, indicating that the latter had better reachability (p < .001) ().
Table 3. Comparison between the two screening methods in the Stockholm cohort.
HCV prevalence
Overall, 235 (35%, 235/676) of the total cohort had a traceable documented anti-HCV test and 11 of them were found to have transfusion-transmitted chronic HCV infection. The prevalence of chronic HCV infection among tested childhood cancer survivors in Stockholm was thus 4.7% (95% CI = 2.6–8.2%), which is almost 10 times higher than the prevalence of 0.5% reported in the adult Swedish population (RR = 9.4) [Citation16]. The HCV prevalence was similar (4.7%, 6/128) among those treated for childhood leukemia before 1992.
Characteristics and treatment outcome of the HCV infected patients
In all, 11 patients were found to be HCV infected (Table S1). All of them were both HCV-antibody and HCV RNA positive. Ten patients were diagnosed before the start of this study. The 11 patients (8 women, 3 men) with chronic HCV infection were treated for malignancy between the years of 1976 and 1990. Blood transfusions during malignancy treatment were the most probable source of HCV infection in all patients. Only two patients were infected after the introduction of surrogate markers for blood donors in 1985. The median age at malignancy diagnosis and treatment was 7 years (range 2–17). The median presumed duration of HCV infection was 22 years at first positive anti-HCV test. Genotype 2 was found in 6 out of 11 (55%). Seven patients underwent liver biopsy at a median duration of infection of 24 years, all of them had mild histopathological signs. Additionally, two patients underwent solely liver elastography with results indicating low fibrosis scores. Nine were successfully treated for chronic HCV infection with sustained viral response (SVR). One patient died from recurrent malignancy before initiation of antiviral treatment.
Discussion
A prevalence of 4.7% chronic HCV infection was found in the HCV tested cohort of childhood cancer survivors in Stockholm, which is almost 10 times higher than the prevalence in the general Swedish population [Citation16]. Previous international studies of transfusion-transmitted HCV infection in childhood cancer survivors have shown a high prevalence, ranging from 6% to 40% depending on location, study period and which malignancies that were included [Citation9–11,Citation17]. All anti-HCV positive patients in our study were also HCV-RNA positive. A high prevalence of seronegative HCV infected childhood cancer survivors has been described previously, suggesting that the true HCV prevalence might have been higher if HCV-RNA was used as screening method instead of anti-HCV [Citation9,Citation18]. On the other hand, the high prevalence of seronegative HCV infections was based on old studies using second generation ELISA, which is a less sensitive anti-HCV detection method. It could also be argued that patients should have restored immunocompetence many years after cessation of treatment and would, therefore, be able to produce antibodies against HCV.
The most common malignancy in our HCV-positive group was leukemia, which was expected since almost all these patients require blood transfusions during treatment [Citation7,Citation8]. Patients treated for brain tumors were in majority among those that never answered the letters, possibly because transfusion rates are lower during brain tumor treatment. Furthermore, patients with highly malignant brain tumors often suffer from posttreatment cognitive side effects, which also might contribute to a lower frequency of study participation [Citation19,Citation20].
We found a high proportion of genotype 2 among the infected childhood cancer survivors. The majority was transfused in the 1980s. Overall, genotypes 1 and 3 are the most frequent genotypes found in Swedish HCV-infected patients [Citation21]. Among patients with transfusion-transmitted HCV infection, genotype 1a is most common, followed by genotype 2b [Citation21–23], whereas genotype 3a is mainly associated with injecting drug use. Shev et al. found a 21% prevalence of genotype 2b among Swedish blood donors in the early 1990s. However, genotype 2b was the most frequent genotype (found in 46%) among blood donors with HCV infection acquired by previous transfusion, suggesting that this genotype used to be more common among blood donors in the 1970–80s [Citation24]. This was confirmed by Westin [Citation21] investigating the distribution of genotypes in Sweden over time, showing that genotype 2 was the second most common genotype before 1975 and thereafter genotype 3a increased in relative prevalence.
Despite a long duration of infection at HCV diagnosis and the additive effect of cytotoxic and hepatotoxic treatments and in some cases also immunosuppressive treatment, the liver disease was mild to moderate among the HCV infected and most patients cleared the infection after treatment. Many previous studies support the finding of slow progression of liver disease among childhood cancer survivors [Citation10,Citation25,Citation26]. However, some patients do develop a more severe liver disease [Citation11,Citation27,Citation28]. Castellino et al. studied 122 childhood cancer survivors at St Jude hospital, USA, of whom 8 of 59 liver biopsied (13.6%) had liver cirrhosis at a median duration of HCV infection of 12.4 years. In addition, they found an association between antimetabolite treatment and a more progressive liver disease [Citation11]. The same cohort of HCV positive childhood cancer survivors was followed until 30 years after cancer diagnosis and the incidence of cirrhosis development was equal to the general HCV infected adult population, but patients developed cirrhosis at a younger age (30 years, median age) [Citation28].
Adult childhood cancer survivors were one of the specifically targeted risk groups in the Swedish national HCV screening campaign during 2007–2010 aimed at blood transfusion recipients between 1965 and 1991. A national prevalence of 0.9% anti-HCV positive and 0.6% HCV-RNA positive was detected in the campaign [Citation14]. Separate analysis of the campaign outcome in Stockholm County showed a higher prevalence of 1.8% anti-HCV positive and 1.4% HCV-RNA positive transfusion recipients [Citation15]. The majority of the HCV positive patients was women infected by blood transfusion during childbirth. Only a few HCV-RNA positive patients were from the targeted pediatric risk groups.
When comparing the effectiveness of the different screening methods used in the Stockholm cohort, active tracing screening was a far more effective method reaching 40% compared to 12% by non-active tracing screening. However, active tracing screening is more time consuming and consequently more expensive. A meta-analysis performed by Aspinall et al. [Citation29] showed that targeted case finding was more effective in test uptake and cases detected, compared to non-targeted testing intervention via public information. This finding is in line with our results and supported by other studies [Citation30,Citation31]. In the era of highly effective direct antiviral treatment options, it is essential to trace and treat undiagnosed HCV infected individuals as soon as possible before progression to severe liver disease. The awareness of previous transfusion history and previous HCV testing is low (around 50%) among childhood cancer survivors [Citation32]. Chronic HCV infection is often asymptomatic, making active screening of specific risk groups the best way to detect previously undiagnosed cases.
Our study has some limitations. The exact number of tested individuals was not possible to estimate since tests performed outside Stockholm County were not possible to track. The prevalence of 4.7% of chronic HCV infection in our cohort is estimated only on the tested population. We were not able to confirm if all included patients had been transfused, since the register lacked information of previous transfusions and no digitalized transfusion records were available during that time period. The prevalence would probably be higher if only those with previous blood transfusions were included. However, childhood cancer survivors undergoing intense medical treatment were also at risk of acquiring HCV infection by the use of unsterilized medical equipment before 1992, and this transmission route would have been neglected if only transfused patients were included [Citation10,Citation33].
In conclusion, we found an almost 10 times higher prevalence of HCV infection among tested childhood cancer survivors in Stockholm, compared to the national prevalence of 0.5%. Only a minority of this risk group had previously been tested for HCV. Assumedly, many transfusion recipients at risk for HCV infection remain untested. With today’s effective treatment options and based on our findings, active tracing and HCV screening of all childhood cancer survivors, who underwent malignancy treatment before 1992, is recommended.
Supplemental Material
Download Zip (162.6 KB)Acknowledgements
The authors would like to thank Petra Edström for digitalizing the hand written pediatric oncology register, and to dr Ulf Hammar for help with statistical analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832.
- Denniston MM, Klevens RM, McQuillan GM, et al. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology. 2012;55:1652–1661.
- Global hepatitis report 2017. Geneva: World Health Organization; 2017.
- Engle RE, Bukh J, Alter HJ, et al. Transfusion-associated hepatitis before the screening of blood for hepatitis risk factors. Transfusion. 2014;54:2833–2841.
- Aach RD, Szmuness W, Mosley JW, et al. Serum alanine aminotransferase of donors in relation to the risk of non-A,non-B hepatitis in recipients: the transfusion-transmitted viruses study. N Engl J Med. 1981;304:989–994.
- Stevens CE, Aach RD, Hollinger FB, et al. Hepatitis B virus antibody in blood donors and the occurrence of non-A, non-B hepatitis in transfusion recipients. An analysis of the Transfusion-Transmitted Viruses Study. Ann Intern Med. 1984;101:733–738.
- Lieberman L, Liu Y, Portwine C, et al. An epidemiologic cohort study reviewing the practice of blood product transfusions among a population of pediatric oncology patients. Transfusion. 2014;54:2736–2744.
- Michon J. Incidence of anemia in pediatric cancer patients in Europe: results of a large, international survey. Med Pediatr Oncol. 2002;39:448–450.
- Arico M, Maggiore G, Silini E, et al. Hepatitis C virus infection in children treated for acute lymphoblastic leukemia. Blood. 1994;84:2919–2922.
- Cesaro S, Petris MG, Rossetti F, et al. Chronic hepatitis C virus infection after treatment for pediatric malignancy. Blood. 1997;90:1315–1320.
- Castellino S, Lensing S, Riely C, et al. The epidemiology of chronic hepatitis C infection in survivors of childhood cancer: an update of the St Jude Children's Research Hospital hepatitis C seropositive cohort. Blood. 2004;103:2460–2466.
- Norda R, Duberg AS, Sonnerborg A, et al. Transmission of hepatitis C virus by transfusion in Orebro County, Sweden, 1990-1992. Scand J Infect Dis. 1995;27:449–452.
- Foberg U, Ekermo B, Widell A, et al. Hepatitis C virus transmission, 1988-1991, via blood components from donors subsequently found to be anti-HCV-positive. Scand J Infect Dis. 1996;28:21–26.
- Duberg AS, Hansdotter F, How AL, et al. [Important with generous sampling for hepatitis C after blood transfusion. The National Board of Health and Welfare's new recommendation for risk groups]. Lakartidningen. 2013;110:1477–1479.
- Millbourn C, Psaros Einberg A, Lindh G, et al. Prevalence and outcome of post-transfusion hepatitis C acquired at different ages and detected in look-back screening. Scand J Gastroenterol. 2018;53:870–875.
- Duberg A, Janzon R, Back E, et al. The epidemiology of hepatitis C virus infection in Sweden. Euro Surveill. 2008;13:1–5.
- Strickland DK, Riely CA, Patrick CC, et al. Hepatitis C infection among survivors of childhood cancer. Blood. 2000;95:3065–3070.
- Locasciulli A, Testa M, Pontisso P, et al. Prevalence and natural history of hepatitis C infection in patients cured of childhood leukemia. Blood. 1997;90:4628–4633.
- Gehrke AK, Baisley MC, Sonck AL, et al. Neurocognitive deficits following primary brain tumor treatment: systematic review of a decade of comparative studies. J Neurooncol. 2013;115:135–142.
- Boman KK, Hoven E, Anclair M, et al. Health and persistent functional late effects in adult survivors of childhood CNS tumours: a population-based cohort study. Eur J Cancer. 2009;45:2552–2561.
- Johan Westin MLLM. Chronic hepatitis C in Sweden: genotype distribution over time in different epidemiological settings. Scand J Infect Dis. 2009;31:355–358.
- Ederth J, Jern C, Norder H, et al. Molecular characterization of HCV in a Swedish county over 8 years (2002-2009) reveals distinct transmission patterns. Infect Ecol Epidemiol. 2016;6:30670.
- Widell A, Shev S, Mansson S, et al. Genotyping of hepatitis C virus isolates by a modified polymerase chain reaction assay using type specific primers: epidemiological applications. J Med Virol. 1994;44:272–279.
- Shev S, Widell A, Foberg U, et al. HCV genotypes in Swedish blood donors as correlated to epidemiology, liver disease and hepatitis C virus antibody profile. Infection. 1995;23:253–257.
- El-Raziky MS, Halawa EF, Draz IH, et al. Natural history and response to treatment of HCV infection among Egyptian survivors of childhood malignancy. Pediatr Hematol Oncol. 2015;32:138–145.
- Fioredda F, Moser A, Bertoluzzo L, et al. Natural course of HCV infection in childhood cancer survivors. Support Care Cancer. 2010;18:1413–1420.
- Strickland DK, Jenkins JJ, Hudson MM. Hepatitis C infection and hepatocellular carcinoma after treatment of childhood cancer. J Pediatr Hematol Oncol. 2001;23:527–529.
- Stallings-Smith S, Krull KR, Brinkman TM, et al. Long-term follow-up for incident cirrhosis among pediatric cancer survivors with hepatitis C virus infection. J Clin Virol. 2015;71:18–21.
- Aspinall EJ, Doyle JS, Corson S, et al. Targeted hepatitis C antibody testing interventions: a systematic review and meta-analysis. Eur J Epidemiol. 2015;30:115–129.
- Jones L, Bates G, McCoy E, et al. Effectiveness of interventions to increase hepatitis C testing uptake among high-risk groups: a systematic review. Eur J Public Health. 2014;24:781–788.
- Cullen BL, Hutchinson SJ, Cameron SO, et al. Identifying former injecting drug users infected with hepatitis C: an evaluation of a general practice-based case-finding intervention. J Public Health (Oxf). 2012;34:14–23.
- Lansdale M, Castellino S, Marina N, et al. Knowledge of hepatitis C virus screening in long-term pediatric cancer survivors: a report from the Childhood Cancer Survivor Study. Cancer. 2010;116:974–982.
- Widell A, Christensson B, Wiebe T, et al. Epidemiologic and molecular investigation of outbreaks of hepatitis C virus infection on a pediatric oncology service. Ann Intern Med. 1999;130:130–134.
