Abstract
Objective: The purpose of this systematic review was to investigate the incidence and nature of minor adverse events (MAEs) after colonoscopy, and response rates to questionnaires concerning MAEs in patients undergoing colonoscopy.
Materials and methods: A systematic literature search was conducted in the databases PubMed and Embase. Predictor variables were patient-reported MAEs after colonoscopy. The outcome was frequency and types of MAEs and the patients’ response rate to questionnaires after colonoscopy. Quality assessment for potential risk of bias and level of evidence was evaluated using the National Health and Medical Research Council guidelines.
Results: Seven prospective cohorts were included with a pooled total of 6172 participants. Patients undergoing colonoscopy had a response rate to questionnaires ranging from 64% to 100%, with a mean of 81%. One-third of the patients experienced MAEs, most prominently in the first 1–2 weeks after colonoscopy, and less common at 30 days post colonoscopy. The most frequently reported MAEs were abdominal pain, bloating and abdominal discomfort.
Conclusions: In general, patients undergoing colonoscopy have a high response rate to questionnaires about MAEs. MAEs after colonoscopy are commonly seen. High age and score of American Society of Anesthesiologists (ASA) classification, female gender and duration of procedure seem to be associated with a higher risk of MAEs, whereas adequate sedation seems to decreases the risk. MAEs after colonoscopy seems to be underreported in the current literature and the existing evidence is based on inhomogeneous reports. In the current study, it was not possible to conduct a meta-analysis. There is a need for larger scale studies addressing the MAEs patients experience in conjunction with a colonoscopy. Furthermore, the assessment of the MAEs should rely on questionnaires tested for validity, comprehensibility and reliability, to reflect the patient-reported experience of a colonoscopy as precise as possible.
Introduction
There has been a dramatic global increase in the numbers of performed colonoscopies, especially after the introduction of bowel cancer screening [Citation1]. Although colonoscopy is regarded as a safe procedure, like other invasive procedures it is still associated with adverse events. Severe complications after colonoscopy such as bowel perforation, bleeding, infection and post-polypectomy syndrome are some of the most reported major adverse events.
A systematic review from the Netherlands on major complications after colonoscopy found a mortality rate of 2.9/100,000 colonoscopies [Citation1]. Even though gastrointestinal endoscopic societies around the world have adopted safety standards for colonoscopy practice, the rates of both major and minor adverse events (MAEs) have been underreported. The assessment of the true frequency of endoscopy-related complications is difficult due to a lack of consensus on a definition. Some define complications as any deviation from the optimal course for the patient after colonoscopy, others define an adverse event if a second intervention is necessary [Citation2,Citation3].
Based on the definitions, a quantification of patient-reported outcomes after a colonoscopy are difficult due to the individual variations in the experience of a colonoscopy, hence the underreporting of patients reported adverse events in current literature.
Furthermore, most of the existing studies have been retrospective and relying on data from endoscopy reports, rather than patient-reported information making the conclusions inhomogeneous and even non-representative of patient experienced adverse events [Citation4].
We find the adverse symptoms that patients may experience adjunct to a colonoscopy of importance. Even if they are referred to as MAEs they might have serious personal consequences for the patients in terms of physical and mental stress [Citation5].
The objective of this review is to investigate the incidence and types of MAEs after colonoscopy, and response rate to questionnaires on patient experienced MAEs after colonoscopy.
Material and methods
We followed the PRISMA methodology for conducting and reporting a systematic review and meta-analysis [Citation6].
Eligibility criteria
Studies were selected according to the criteria outlined below.
We aimed to include studies examining a representative adult human population (18 years or older). There were no restrictions regarding sample size. The interventions of interest were colonoscopy and patient-reported MAEs. We wanted to include studies addressing both colonoscopy and esophagogastroduodenoscopy (EGD) if data provided for colonoscopy was reported separately. Outcomes were collected as the rate and type of MAEs after colonoscopy and patients’ response rate to questionnaires. We would include MAEs, defined as any health problem experienced during the study period after the procedure, which did not lead to hospitalization. Non-manageable bleeding during the procedure, bleeding that led to hospital or outpatient clinic visits, and bowel perforations that were not immediately handled during the colonoscopy were classified as major. There were no restrictions on the length of follow-up.
We included articles published on colonoscopy from the last 20 years (January 1998 to February 2018), because of progress in endoscopic equipment and improvement of education and techniques. Articles in English or Scandinavian languages were included.
Search strategy
In order to identify appropriate literature, a structured systematic search was conducted on the 26 February 2018. Databases searched were PubMed and Embase, and the keywords included were MAEs, postoperative complications and colonoscopy. For specific search strings, see Appendix A. Exclusion criteria were major complications, therapeutic colonoscopies (e.g., polypectomy), not-patient-reported complications, EGD or colonoscopies in children. Review articles, case reports, letters and studies investigating treatment were also excluded. Full text of the remaining studies was examined for relevant information.
Data extraction and quality assessment
Pre-specified data were obtained from all seven selected articles including the following parameters: first author and year of publication, location, number of participants, indication for colonoscopy, data collection, response rate, age, gender, operator experience, follow-up time, procedure duration, sedation type and sedation dose ().
Table 1. Characteristics of selected studies.
The articles were assessed using the National Health and Medical Research Council (NHMRC) guidelines for levels of evidence and grades [Citation7]. To facilitate the assessment of the possible risk of bias for each study, we collected information using the Cochrane Collaboration tool, which covers: sequence generation, allocation concealment, blinding, incomplete outcome data and selective outcome reporting [Citation8].
If studies were sufficiently homogenous in terms of design and comparator, we could conduct a meta-analysis.
Endpoints
The primary endpoint was to estimate the types and rates of MAEs after colonoscopy. The secondary endpoint was to investigate response rates to different methods of obtaining the patient-reported data after colonoscopy.
Results
Systematic search
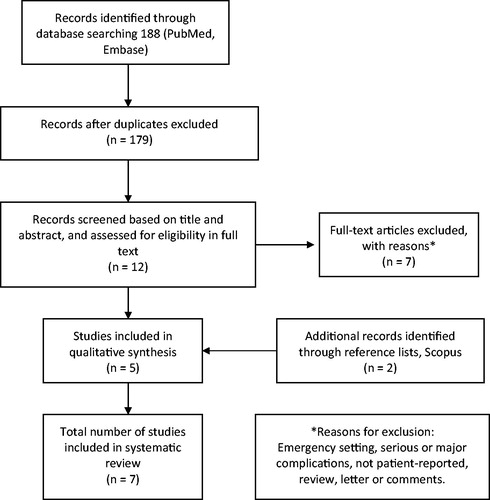
The search strategy and selection of studies are shown in a flow diagram in . After excluding duplicates, 179 articles were found initially in PubMed and Embase. Twelve were relevant after reading the title and abstract. After excluding seven articles, the remaining five [Citation9–13] were read thoroughly by all authors. To ensure literature saturation, a reference search was conducted on Scopus based on the included articles, where two additional studies [Citation2,Citation14] were found. The systematic search yielded seven studies with a total study population of 6172 patients [Citation2,Citation9–14]. The colonoscopies were performed over a period from January 1997 to June 2014.
Study characteristics
All seven studies were prospective cohort studies, with level III of evidence. Mean age of the study populations varied from 50 to 59 years. All studies included had an even distribution of gender (female participants ranging from 46% to 55%). Two studies reported solely on screening and surveillance colonoscopies [Citation9,Citation14]. In the study of Bini et al. [Citation10], they evaluated complications related to endoscopy in a training setting, while the other studies included varying levels of endoscopy experience. The mean duration of the colonoscopies in the studies ranged from 10.8 to 29.5 min, however not all stated this parameter. The types of sedation used were Midazolam, Fentanyl, Alfentanil and Pethidine. Most commonly used was Midazolam, where the mean use ranged from 3.1 to 4.8 [Citation9,Citation11,Citation13,Citation14].
Study methodologies differed in terms of questionnaires, timing and collection of response. The timing of questionnaires varied from during the stay at the unit [Citation11] to 24 h [Citation14], 48 h [Citation13], 7 days [Citation9], 14 days [Citation13] and 30 days [Citation9,Citation11–14]. The type of survey varied across studies between using telephone interviews [Citation9,Citation11,Citation12,Citation14] and/or e-mail questionnaires [Citation13] to obtain information on patients’ perspectives. The response rate ranged from 64% to 100% at the last follow-up consultation. Bini et al. [Citation10] (30 days follow-up), Ko et al. [Citation9] (7 and 30 days follow-up) and Marquez et al. [Citation13] (2, 14 and 30 days follow-up) differed between early and late MAEs in their interviews. De Jonge and Zubarik (both 30 days follow-up) did not specify and could have been MAEs experienced by the patient in any time between the colonoscopy and 30 days follow-up [Citation2,Citation12]. Lee et al. [Citation14] reported only early MAEs (24 h).
Zubarik et al. [Citation2] was the earliest study found in the literature on the topic. In 1999, they enrolled 1126 patients in an MAEs study after colonoscopy and compared sedated and non-sedated patients. They contacted the participants 30 days after the procedure by telephone and 74% agreed to participate. Patients reported an MAEs total of 16%. From the colonoscopies alone (EGDs were also included in the study), 6% reported abdominal discomfort. The questionnaire contained five questions about: MAEs possibly related to the procedure, if patients went to a doctor or emergency room, hospitalization, if patients were employed or missed time from work. However, it was unspecified if the questions were designed only by the investigators or if they were based on other literature.
Bini et al. [Citation10] evaluated MAEs after 400 colonoscopies done by gastroenterological fellows in a training setting. They either mailed or telephoned patients, and had a response rate to the questionnaires of 86%. They found a total of 15% MAEs 30 days after colonoscopy. The main MAEs were abdominal discomfort (5%), nausea/vomiting (4%) and shortness of breath (4%). The study had a questionnaire with 14 questions. They specifically asked about nausea, vomiting, sore throat, headache and abdominal discomfort.
Lee et al. [Citation14] reported solely on post-colonoscopy abdominal pain, which was present in 49% of the patients. They contacted patients by phone 24 h after the colonoscopy and had a response rate of 100%. The study measured frequency and severity of any mental distress symptom after colonoscopy using the Brief Symptom Rating Scale: 0, not at all; 1, a little bit; 2, moderately; 3, quite a bit; or 4 extremely contributory to distress [Citation15]. The study concluded that female sex and duration of colonoscopy increased the likelihood of post-procedural abdominal pain. They also found that irritable bowel syndrome may lead to prolonged discomfort.
Ko et al. [Citation9] contacted 502 patients by telephone at day 7 and day 30 after colonoscopy, with a 94% response rate. They found that up to 34% of the patients reported at least one minor gastrointestinal symptom 7 days after colonoscopy. The most common MAEs was bloating (25%) and abdominal pain and/or discomfort (5–11%). At day 30, the total rate of MAEs of 6% were reported. The most common MAE at that point was nausea/vomiting (2%). Being female led to a higher risk of reporting MAEs (OR 1.78, 95% CI 1.21–2.62) and longer procedure duration (compared with procedure duration less than 20 min: OR 1.06 [95% CI 0.64–1.75] for 20-29 min, OR 1.77 [95% CI 1.03–3.05] for 30-39 min, OR 2.63 [95% CI 1.49–4.63] for >40 min) was significant risk factors for MAEs. The questionnaire was not included in the article, and the specific questions are unknown. Trained research personnel asked the participants about the development of new symptoms after the procedure, including abdominal pain, GI bleeding and fever. Subjects were specifically asked not to include preexisting GI symptoms, such as abdominal pain or bloating.
Baudet et al. [Citation11] included 1126 patients to determine minor complication rates in outpatient colonoscopy procedures and the effect of sedation on these complications. It is unknown which articles the questionnaire was based upon, and no list of questions was included. Patients were contacted during their stay in the endoscopy unit and 30 days post-procedure by telephone, with a response rate of 81%. MAEs in the early phase were observed in 31% of patients (25% among sedated patients; 52% in non-sedated patients; p < .001); 23% had late MAEs (16% of sedated patients vs. 51% of non-sedated patients; p < .001). The most common MAEs were abdominal pain and distension (15%). The risk of experiencing complications (odds ratio) was 1.013 times higher for high age (CI 95% 1.004–1.022); 1.953 times higher per grade increase in American Society of Anesthesiologists (ASA) classification (95% CI 1.524–2.504); and 0.116 times higher when sedation was used (95% CI 0.079–0.170).
De Jonge et al. [Citation12] contacted patients by phone 30 days after colonoscopy and had a response rate of 75%. MAEs were reported by 41% of the patients. They developed a standard telephone interview based on the most common adverse events reported in the literature. They asked about any kind of gastrointestinal complaint and especially abdominal discomfort, which was present in 17% of patients. They found that female sex (OR 1, 5), age >50 years (OR 1, 5), colonoscopy for colorectal screening/surveillance (OR 1, 6) and fellow-endoscopy (OR 1, 7) were risk factors for the occurrence of MAEs. The study included eight questions; and if patients had any other gastrointestinal complaint, they were asked to elaborate. There were both a pre- and post-colonoscopy questionnaire. There were no significant differences in the characteristics of the responders versus non-responders.
Marquez et al. [Citation13] used telephone or e-mail follow-up to obtain data for 2, 14 and 30 days follow-up. In the end, 64% of participants had responded to all the questionnaires. At baseline, the investigators asked about symptoms present in a 30-day period before the colonoscopy. The MAEs assessed in the questionnaires were: abdominal pain, bloating, diarrhea, constipation, nausea, vomiting, blood in stools, rectal pain and headache. Other MAEs reported by patients were disorientation, fatigue, itchiness, fever and dehydration. However, no appendix of the questionnaire was included; also it is uncertain what literature the questions were based upon. At the 2 days follow-up, 17% reported at least one MAE. At the 14 and 30 days follow-up, 11%, and 3% reported at least one MAE, respectively. Abdominal pain (9%) and bloating (6%) were the most commonly reported on day 2 and 14. However, on day 30, only 1% had abdominal pain ().
Table 2. The tables illustrate, the percentages of the most common MAEs from the selected studies a) 1–14 days and b) 30 days of follow-up.
Discussion
This is to our knowledge the first study to systematically assess the existing literature on patient-reported adverse events after colonoscopy and its suitability for a meta-analysis. Our findings were not in coherence with standards set to perform a meta-analysis because there were no control groups, and the parameters used varied substantially.
We founded an obvious limitation in the inhomogeneous nature of the seven included studies, thus emphasizing the need for studies assessing the underreported rate on MAEs after colonoscopy.
A systematic review [Citation1] has been conducted previously on post-colonoscopy complications. However, they focused on the serious events, and only one study is commonly included with the current analysis. The questionnaires used in the included studies to collect patient-reported complications are generally not well validated. It is generally unknown which articles the questionnaire was based upon. Also, almost half of the included studies did not report on questionnaire details. There is a great variation of time point for feedback, which makes it difficult to define and differentiate between early and late MAEs. Another risk of bias for most studies is the uncertainty whether the MAEs were pre-colonoscopy symptoms.
Minor adverse events
The most common MAEs after colonoscopy seem to be bloating and abdominal pain/discomfort. Marques et al. [Citation13] concluded that MAE rates are common and predominantly occur in the first two weeks post colonoscopy and cause little use of healthcare resources. De Jonge et al. [Citation12] found that patients undergoing colonoscopy for screening or surveillance more often reported adverse events. They hypothesized that patients without symptoms pre-colonoscopy may notice and report MAEs to a higher extent after colonoscopy. The real incidence of adverse events is underestimated both in number and in impact when only the doctors’ reported adverse events are included [Citation12]. Also, if a study screens for many different MAEs, there is a higher possibility of detecting more symptoms. Therefore, the true outcome might be reflected in each parameter enquired, for example, bloating, abdominal pain, abdominal discomfort, nausea, constipation, etc.
Most studies in the review did not account for previously existing symptoms before the colonoscopy, which may result in overestimation of the incident of MAEs [Citation2,Citation10–13]. A general point of limitation concerning the included studies could be recall-bias, if patients were unable to remember MAEs at the 30 days follow-up. Also, there exists a risk of bias that the symptom might not even originate from the colonoscopy, but from something else that had evolved in the meantime. Four studies [Citation2,Citation10–12] contacted patients only once after 30 days, which makes it likely that late interviews for MAEs could be unreliable because of recall-bias. It is unclear if patients in the 30-day follow-up had experienced the MAEs at a certain point after colonoscopy or were still experiencing them.
Only three studies showed a detailed appendix concerning the questionnaires used [Citation2,Citation10,Citation12]. Only de Jonge et al. stated that the development of the questionnaire was based on the most common adverse events reported in the literature [Citation12]. There is a risk of bias for all studies since none of the questionnaires were tested for validity, comprehensibility and reliability.
Patient-centred care models have shifted attention to patient satisfaction and comfort. It is important to address MAEs, which do not result in hospitalization but may cause significant discomfort for the patient, which is a core issue in quality assurance for colonoscopy [Citation12,Citation13].
Colonoscopy procedure
A meta-analysis of 21 RCTs from 2012 found that CO2 insufflation during colonoscopy causes lower post-procedural pain and bowel distension compared to air insufflation [Citation16]. Most studies did not state whether carbon dioxide or atmospheric air were used to insufflate the patients [Citation9–12]. Lee et al. [Citation14] only used atmospheric air insufflation and Marquez et al. [Citation13] insufflated most patients (76%) with carbon dioxide. However, for patients given atmospheric air, Marquez et al. [Citation13] did not find this a predictor of post-colonoscopy discomfort, this could be due to lack of statistical power.
It is not known whether improvements in colonoscopy training and technological progress have led to a change in the post-colonoscopy complication rates [Citation17,Citation18]. All studies but one included in the current study used varying level of experienced gastroenterologists to perform the colonoscopies. Bini et al. [Citation10] used only fellows in a training setting, where serious complications were uncommon, and MAEs occurred in only 14%.
Collection and rates of replies
The population of patients undergoing colonoscopy had a response rate to surveys ranging from 64% to 100%, with a mean of 81%. Most studies collected replies by telephone, and only Marquez et al. [Citation13] collected answers by e-mail and an Internet-based survey. When participants were not reached, they were telephoned daily for the next three consecutive days (one attempt per day). However, in spite of this extensive outreach to patients, they had the lowest response rate (64%) compared to the other studies [Citation13]. Lee et al. had a 100% response rate, but it is unclear how they gained such a high success. The study does not explain if only responders were included in the analysis, or if they had a response from all 1000 participants. Another limitation of Lee et al. [Citation14] is a very short follow-up time (24h) when comparing to the other studies, which all had 30 days. Ko et al. [Citation9] had the highest response rate at 30 days follow-up with 94% replies by telephone contact. Previous studies have been conducted between 1998 and 2014, where online surveys might not have been so common. However, now in 2018, it is easy and time saving to conduct an Internet-based follow-up which opens up the possibilities to conduct studies on larger populations.
Conclusion
The included studies showed that one-third of the patients experienced MAEs, most common in the first 1–2 weeks after colonoscopy, and less common at 30 days post colonoscopy. The majority of reported MAEs were abdominal pain, bloating and abdominal discomfort. High age and ASA score, female sex and procedure duration seem to be risk factors for developing MAEs, whereas sedation lowers the risk. Information about the risk of MAEs is essential to patients both pre- and post-colonoscopy in order to obtain an informed patient consent, where MAEs should not be kept unexpected for patients.
For future studies, we recommend a study population of at least 1000 participants, containing pre-colonoscopy symptoms, with follow-up at 24 h and 30 days to define early and late MAEs.
The questionnaire should be tested for validity, comprehensibility and reliability; preferably by both patients and doctors in advance. To avoid the risk of missing any type of patient-reported MAEs, patients should be given the opportunity to report additional MAEs if they are not already mentioned in the questionnaire.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Reumkens A, Rondagh EJA, Bakker CM, et al. Post-colonoscopy complications: a systematic review, time trends, and meta-analysis of population-based studies. Am J Gastroenterol. 2016;111:1092–1101.
- Zubarik R, Fleischer DE, Mastropietro C, et al. Prospective analysis of complications 30 days after outpatient colonoscopy. Gastrointest Endosc. 1999;50:322–328.
- Johanson JF, Schmitt CM, Deas J, et al. Quality and outcomes assessment in gastrointestinal endoscopy. Gastrointest Endosc. 2000;52:827–830.
- Fleischer DE. Better definition of endoscopic complications and other negative outcomes. Gastrointest Endosc. 1994;40:511–514.
- Steffenssen MW, Al-Najami I, Zimmermann-Nielsen E, et al. Patient-reported complications related to colonoscopy: a prospective feasibility study of an email-based survey. Acta Oncol (Madr). 2018;1–6 [Epub ahead of print].
- Moher D, Liberati ATJA. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:2535–2535.
- NHMRC additional levels of evidence and grades for recommendations for developers of guidelines [Internet]. 2018. Available from: http://www.nhmrc.gov.au/_files_nhmrc/file/guidelines/stage_2_consultation_levels_and_grades.pdf
- Higgins JPT, Altman DG, G⊘tzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Ko CW, Riffle S, Shapiro JA, et al. Incidence of minor complications and time lost from normal activities after screening or surveillance colonoscopy. Gastrointest Endosc. 2007;65:648–656.
- Bini EJ, Firoozi B, Choung RJ, et al. Systematic evaluation of complications related to endoscopy in a training setting: a prospective 30-day outcomes study. Gastrointest Endosc. 2003;57:8–16.
- Baudet JS, Diaz-Bethencourt D, Aviles J, et al. Minor adverse events of colonoscopy on ambulatory patients: the impact of moderate sedation. Eur J Gastroenterol Hepatol. 2009;21:656–661.
- de Jonge V, Nicolaas JS, van Baalen O, et al. The incidence of 30-day adverse events after colonoscopy among outpatients in the Netherlands. Am J Gastroenterol. 2012;107:878–884.
- Marquez Azalgara V, Sewitch MJ, Joseph L, et al. Rates of minor adverse events and health resource utilization postcolonoscopy. Can J Gastroenterol Hepatol. 2014;28:595–599.
- Lee YC, Wang H-P, Chiu HM, et al. Factors determining post-colonoscopy abdominal pain: prospective study of screening colonoscopy in 1000 subjects. J Gastroenterol Hepatol. 2006;21:1575–1580.
- Version E. Brief Psychiatric Rating Scale (BPRS). J Clin Psychol. 1990;46:168–174.
- Wang WL, Wu ZH, Sun Q, et al. Meta-analysis: the use of carbon dioxide insufflation vs. room air insufflation for gastrointestinal endoscopy. Aliment Pharmacol Ther. 2012;35:1145–1154.
- Anderloni A, Jovani M, Hassan C, et al. Advances, problems, and complications of polypectomy. Clin Exp Gastroenterol. 2014;7:285–296.
- Iqbal CW, Chun YS, Farley DR. Colonoscopic perforations: a retrospective review. J Gastrointest Surg. 2005;9:1229–1236.
Appendix A. Article search history
The appendix illustrates the study search strategies. It is separated in columns with the search strategies and rows depending on the search engine used.