Abstract
Background: Cancer is currently one of the most important factors affecting the long-term health and survival of heart transplant patients.
Material and methods: We calculated the standardized incidence ratios (SIR) for different cancer sites and the cancer-specific standardized mortality ratio (SMR) by linking a cohort of 479 adult heart transplant recipients transplanted in 1985–2014 (4491.6 person-years of follow-up) with data from the national Finnish Cancer Registry until the end of 2015, and with the data from the Statistics Finland’s national registry of causes of death.
Results: A total of 267 cancers occurred in 143 patients (SIR 6.0; 95% confidence interval (CI) 5.3–6.7). The SIR for overall cancer was considerably higher for men (SIR 6.7; 95% CI 5.9–7.5) than for women (1.4; 95% CI 0.6–2.6). Most frequent cancers were non-melanoma skin cancers (basal cell carcinoma 83 cases, squamous cell skin cancer (SCC) 56 cases), followed by Non-Hodgkin lymphoma (NHL) (36 cases), lung cancer (17), cancer of prostate (16) and cancer of kidney (12). SIRs were highest for SCC (51.9; 95% CI 39.2–67.4), lip cancer (47.4; 95% CI 19.1–97.7), cancer of tongue (26.3; 95% CI 7.2–67.4), and NHL (25.7; 95% CI 18.0–35.6). For most cancers, SIRs increased steadily by time since transplantation. Cancer mortality was three times higher for heart transplant recipients than for the population (SMR 3.1; 95% CI 2.1–4.1).
Conclusions: Both cancer incidence and mortality are remarkably increased after heart transplantation, with the relative incidence most elevated for SCC, lip and other oral cancers, and for NHL.
Introduction
As the short-term survival after heart transplantation (HTx) has improved considerably during recent decades, cancer as a later complication has become one of the most important causes of morbidity and mortality among heart transplant recipients. According to the statistics of The International Society for Heart and Lung Transplantation (ISHLT), 16% of all 5-year-survivors and 28% of 10-year-survivors have been diagnosed with at least one post-HTx cancer. Furthermore, beyond five years after HTx, cancer is currently the leading cause of death [Citation1,Citation2]. For patients transplanted since the year 2000, the risk of developing a cancer 1–5 years after HTx has been estimated to be 10–12% [Citation3].
Compared to the normal population, cancer risk after solid organ transplantation has been found two to four times higher, and more elevated in thoracic organ than liver or kidney transplant recipients [Citation4–10]. Increased incidence of cancer after transplantation is considered to relate to the immunosuppression [Citation10–12]. In this epidemiological study, we assessed the cancer incidence and cancer mortality in Finnish heart transplant recipients in comparison to the general Finnish population.
Material and methods
Cohort, data acquisition and statistical analysis
The cohort included 479 subsequent adult primary heart transplant recipients, starting from the first HTx in Finland in 1985 until the end of 2014. Patients who had received their first transplant in childhood were excluded. Patients with re-transplants were not excluded when the primary transplant had been received as adult; the follow-up for cancer started from the first transplant. None of the patients had received other transplant before heart transplantation. Clinical data (patients’ age, gender and indication for transplantation) were collected retrospectively from Helsinki University Hospital’s records. Characteristics of the cohort are shown in . The study was approved by the Ethics Committee of Helsinki University Hospital and by the National Institution for Health and Welfare.
Table 1. Characteristics of the cohort.
Vital status of all patients at the end of the follow-up and the date and cause of death for deceased patients were searched from Statistics Finland’s official national register of causes of death with the patients’ official personal identity codes (given to all residents of Finland and used in all official national registers). The death was counted as cancer-attributable when the official cause of death (classified with ICD-10 codes from 1996 and with ICD-8 codes from the beginning of the follow-up of the cohort in 1985 until 1995) was cancer. All data on both pre-transplant and post-transplant cancers were retrieved using the patients’ official personal identity codes from the Finnish Cancer Registry, which has recorded all cancer cases detected in Finland since 1953 [Citation13]. Finnish Cancer Registry also records basal cell carcinoma, though it is not included in the official statistics and the way of recording the cases have varied during the decades [Citation13].
Cancer follow-up started on the date of heart transplantation and ended at the patient’s death or on 31 December 2015, whichever came first. The expected numbers of cancer cases and cancer deaths were calculated by multiplying the number of person-years at risk by the corresponding cancer incidence and mortality rate in the year-, age- and gender-matched Finnish population. The standardized incidence ratios (SIR) and the standardized mortality ratios (SMR) were calculated by dividing the observed number of cases by the expected number of cases based on the population incidence recorded be the Finnish Cancer Registry. The 95% confidence intervals (CI) for the SIRs and SMRs were calculated based on the assumption that the number of observed cases followed a Poisson distribution. p-values <.05 were considered statistically significant. The absolute excess risk (AER) was calculated by subtracting the expected number of cases from the observed number of cases and dividing by the person-years at risk.
Clinical follow-up and immunosuppression protocol
Transplantations in Finland are performed only in Helsinki University Hospital. HTx patients’ clinical follow-up during the first post-HTx year is done in the transplant centre; thereafter stable patients are followed in university hospitals according to patients’ domicile. Cancer surveillance is performed as a part of the clinical monitoring including regular laboratory tests and chest x-ray once a year. Patients’ skin is checked yearly, and the patient is referred to a dermatologist if any changes are detected.
All patients received immunosuppression based on calcineurin-inhibitors (cyclosporine for patients transplanted before 2008, thereafter mainly tacrolimus) combined with either azathioprine (HTx before 2002) or mycophenolate mofetil (HTx in 2002 and later). Induction immunosuppression therapy consisted of intravenous methylprednisolone at transplantation followed by oral methylprednisolone for 6–12 months for all patients. In addition, until the end of 2010, polyclonal anti-thymocyte antibodies (anti-thymocyte globulin; ATG) were administrated during the first three days for all patients, and thereafter only for patients considered to be at high risk of acute rejection. Patients with giant cell myocarditis, multiple rejections, or autoimmune diseases received oral methylprednisolone beyond one year from transplantation. Rejections were treated by raising the oral methylprednisolone dose in cases of persistent or recurrent class 1R rejections and with intravenous high-dose steroids in class 2R and 3R rejections.
Results
Cohort
A total of 4491.6 person-years of follow-up were recorded from the 479 patients. Of all patients, 415 (86.6%) were alive at 30 days from the transplantation and 386 (80.6%) at one year. At the end of the follow-up 234 (48.9%) patients were alive. The median length of follow-up was 7.8 years (interquartile range (IQR) 13.0 years). Median age at transplantation was 52 years (IQR 15.0) and 79.5% of the patients were men.
Cancer incidence
In total, 267 post-transplant cancers occurred in 143 patients during the follow-up. () The cumulative cancer incidence in the survivors at 1, 5, 10 and 20 years was 0.3%, 8.7%, 22.3% and 52.4%, respectively. Of all cancers, 96.3% occurred in men. Median time from transplantation to any cancer was 8.9 years (IQR 8.9 years), to a solid organ cancer 9.8 years (IQR 9.1 years), to non-melanoma skin cancer (NMSC) 9.8 years (IQR 9.6 years), and to a Hodgkin or non-Hodgkin lymphoma 9.0 years (IQR 9.3 years). Of the 21 patients with history of pre -HTx cancer, 11 (52.0%) were diagnosed with a de-novo cancer after HTx. No relapses of prior cancer occurred.
Table 2. All cancers with their corresponding SIRs.
Of all cancers detected in the cohort, most frequent were NMSC (basal cell carcinoma 83 cases, 31.1% of all cancers in the cohort; SIR 10.5, and squamous cell cancer 56 cases; 21.0%; SIR 51.9), followed by NHL (36; 13.5%; SIR 25.7). Of the solid organ cancers, most common were lung cancer (17 cases; 6.4%; SIR 3.9), cancer of prostate (16; 6.0%; SIR 1.5) and cancer of kidney (12; 4.5%; SIR 9.5) (). SIR for overall cancer in the whole cohort was 6.0 (95% CI 5.3–6.7) (), and excluding NMSCs, 3.6 (95% CI 3.0–4.2). The overall SIR for men was four times higher (6.7; 95% CI 5.9–7.5) than for women (1.4; 95% CI 0.6–2.6). Of different cancer sites with at least two detected cases, SIRs were highest for SCC (SIR 51.9; 95% CI 39.2–67.4), lip cancer (47.4; 95% CI 19.1–97.7), cancer of tongue (26.3; 95% CI 7.2–67.4), and NHL (25.7; 95% CI 18.0–35.6) ().
The overall SIR was elevated from the beginning of the follow-up and further increased by time since transplantation (). For most cancer sites, the SIR was most elevated beyond 10 years from HTx. For non-Hodgkin lymphoma (NHL), SIR was highly elevated (24.4; 95% CI 3.0–88.2) already from the first year after transplantation, further increasing to 31.0 (95% CI 15.6.57.1) at 10 to 15 years after transplantation. SIR for overall cancer was highest in patients under 40 years at transplantation (SIR 15.4; 95% CI 10.8–21.1), decreasing by age at HTx: 9.2 (95% CI 7.3–11.4) for patients 40 to 49 years, 4.8 (95% CI 4.0–5.8) for patients 50 to 59 years, and 3.7 (95% CI 2.6–5.0) for patients ≥60 years.
Figure 1. Standardized incidence ratio (SIR) for overall cancer with 95% confidence intervals as a function of time since heart transplantation (HTx).
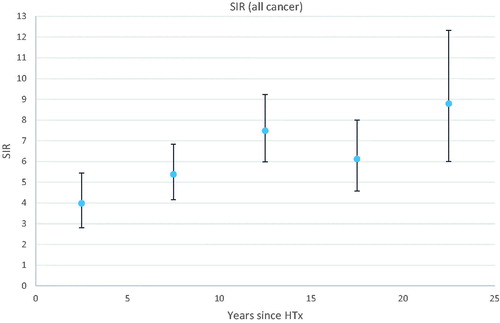
The AER of overall cancer including NMSCs was 49.5/1000 person-years at risk, and excluding NMSCs, 19.7/1000 person-years. According to the age at HTx, AER was 31.7/1000 person-years for patients <40 years old; 57.0/1000 person-years for patients 40 to 49 years; 53.2/1000 person-years for patients 50 to 59 years, and 51.2/1000 person-years for patients ≥60 years.
Cancer mortality
Cancer was classified as the cause of death in 52 patients, representing 21.2% of all deaths in the cohort during the follow-up. During the first post-HTx year, only two cancer deaths occurred, and in the first five years after HTx, nine. The cancer-specific SMR for the whole cohort was 3.1 (95% CI 2.4–4.1), increasing slowly over time since HTx: 2.3 (95% CI 0.8–4.9) in first five years from HTx, 3.0 (95% CI 1.6–5.0) at 5–10 years, 3.3 (95% CI 2.2–4.8) at 10–20 years and 4.6 (95% CI 2.0–8.8) beyond 20 years after HTx. The cancer-specific SMR was more elevated for men (3.3; 95% CI 2.5–4.3) than for women (1.8; 95% CI 0.5–4.7), and highest for young patients: 8.0 (95% CI 2.5–18.6) for patients <40 years at transplantation, 5.8 (95% CI 3.3–9.3) for 40–49 years, 2.0 (95% CI 1.3–3.2) for 50–59 years, and 3.2 (95% CI 1.8–5.2) for ≥60 years at transplantation.
Discussion
Our study demonstrates that the cancer incidence in Finnish HTx-recipients is six times higher than in the general Finnish population. None of the cancer sites shows significantly decreased SIRs, and the absolute excess risk is high in all categories. The overall SIR is considerably higher in younger patients compared to the older, and in men compared to women, as usual in post-transplant cohorts [Citation6,Citation14–16]. Cancer mortality after HTx is three-fold compared to the population, though on the same level as reported on other HTx cohorts [Citation17,Citation18].
The overall SIR of 6.0 in our study was higher than reported in most previous population-based studies on HTx-cohorts: SIR 2.7 for a Canadian cohort [Citation16], 2.6 for an Australian cohort [Citation19], and 2.5 in a large UK registry study [Citation14]. Noteworthy, all these studies excluded NMSCs, leaving the overall SIR 3.6 excluding NMSCs in our cohort actually on the same level [Citation14,Citation16,Citation19]. In a large Swedish study on a pooled post-transplant cohort of solid organ recipients, the overall SIR including SCC for thoracic transplant (heart and/or lung) recipients was as high as 10, and 3.3 excluding SCC [Citation20].
NMSCs were the most frequent cancer in our cohort, covering more than half of all the cancers detected. The relative risk was highest for SCC with a 52-fold incidence, basal cell skin cancer also having a 10-fold incidence. Other frequent cancers in our cohort were cancers that are common also in the Finnish population [Citation21]: cancer of prostate, lung cancer and cancer of kidney. However, the population-compared incidence was significantly elevated for all of them except for cancer of prostate. As expected, NHL was numerous with 36 cases (SIR 25.7), with the same level of excess incidence than in the Swedish cohort [Citation20]. Other cancers with highly elevated SIRs were cancers of the lip and tongue (SIR 47.4 and 26.3, respectively).
Our findings seem to follow the same pattern reported before on the excess cancer incidence in thoracic organ recipients, with most increased incidence for NMSCs, especially for SCC, and other virus-associated cancers [Citation14,Citation20,Citation22–26]. Kaposi’s sarcoma, however, occurred in only one patient in our cohort. As part of oral cancers are associated with human papilloma viruses [Citation27,Citation28], the reduced viral control caused by immunosuppression is considered to be one reason why transplant recipients are more susceptible to these cancers [Citation29]. Papilloma viruses have also been suggested to contribute to the increased rate of SCC in transplant recipients [Citation30,Citation31]. The use of ATG in immunosuppression has been considered to increase the risk of lymphomas and skin cancers [Citation10,Citation32–34]. In recent years, coinciding with the declining use of ATG, a decrease in lymphoma incidence after both heart and lung transplantation has been reported [Citation35,Citation36]. Unfortunately, we were unable to compare the incidence of NHL by the immune suppression protocols used due to insufficient follow-up time for the later strata.
The main limitation of our study is the relatively small cohort even though the follow-up time is long, resulting in small number of cases for many of the cancer sites. Also, we were unable to compare long-term cancer risk in different immunosuppressant protocol eras, as the current protocol has been used only since 2011. The strength of our study is the comprehensive registers. Since 1953 Finnish hospitals, private physicians and laboratories have been obligated to report all detected cancers to the Finnish Cancer Registry. Cancer data is also received via death certificates. Therefore, the Finnish Cancer registry’s database covers nearly 100% of cancers observed in Finland [Citation13]. All transplantations in Finland are performed in Helsinki University Hospital, which ensures that the study includes all adult HTx recipients since 1985.
Conclusions
Our results demonstrate that both cancer incidence and cancer mortality in Finnish heart transplant recipients are significantly elevated. Men are at remarkably higher risk than women. The risk for cancer is already increased in the first years after HTx and further increases over time. NMSCs are the cancers occurring most frequently, highlighting the importance of regular skin checkup, especially as NMSCs tend to behave more aggressively in transplant recipients than in other patients [Citation31]. Further studies are needed to determine if the cancer risk after HTx decreases due to the lower immunosuppression used in recent years, and to identify other possible risk factors for cancer after HTx.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Khush KK, Cherikh WS, Chambers DC, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-fifth adult heart transplantation report—2018; focus theme: multiorgan transplantation. J Heart Lung Trans. 2018;37:1155–1168.
- ISHLT Adult Heart Transplantation Statistics. 2018. [cited 2018 Dec 4]. Available from: https://ishltregistries.org/registries/slides.asp.
- Youn J, Stehlik J, Wilk AR, et al. Temporal trends of de novo malignancy development after heart transplantation. J Am Coll Cardiol. 2018;71:40–49.
- Åberg F, Pukkala E, Höckerstedt K, et al. Risk of malignant neoplasms after liver transplantation: A population-based study. Liver Trans. 2008;14:1428–1436.
- Adami J, Gabel H, Lindelof B, et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003;89:1221–1227.
- Engels EA, Pfeiffer RM, Fraumeni JF, et al. Spectrum of cancer risk among US solid organ transplant recipients. Jama. 2011;306:1891–1901.
- Kyllonen L, Salmela K, Pukkala E. Cancer incidence in a kidney-transplanted population. Transpl Int. 2000;13:394.
- Hall EC, Pfeiffer RM, Segev DL, et al. Cumulative incidence of cancer after solid organ transplantation. Cancer. 2013;119:2300–2308.
- Doesch AO, Müller S, Konstandin M, et al. Malignancies after heart transplantation: incidence, risk factors, and effects of calcineurin inhibitor withdrawal. Transplant Proc. 2010;42:3694–3699.
- Crespo-Leiro MG, Alonso-Pulpón L, Vázquez de Prada JA, et al. Malignancy after heart transplantation: incidence, prognosis and risk factors. Am J Transplant. 2008;8:1031–1039.
- Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. 2005;80:254.
- Vajdic CM, van Leeuwen MT. Cancer incidence and risk factors after solid organ transplantation. Int J Cancer. 2009;125:1747–1754.
- Pukkala E, Engholm G, Højsgaard Schmidt LK, et al. Nordic cancer registries - an overview of their procedures and data comparability. Acta Oncol. 2018;57:440–455.
- Collett D, Mumford L, Banner NR, et al. Comparison of the incidence of malignancy in recipients of different types of organ: a UK registry audit. Am J Trans. 2010;10:1889–1896.
- Higgins RS, Brown RN, Chang PP, et al. A multi-institutional study of malignancies after heart transplantation and a comparison with the general United States population. J Heart Lung Trans. 2014;33:478–485.
- Jiang Y, Villeneuve PJ, Wielgosz A, et al. The incidence of cancer in a population-based cohort of Canadian heart transplant recipients. Am J Trans. 2010;10:637–645.
- Acuna SA, Fernandes KA, Daly C, et al. Cancer mortality among recipients of solid-organ transplantation in Ontario, Canada. JAMA Oncol. 2016;2:463–469.
- Na R, Grulich AE, Meagher NS, et al. De novo cancer-related death in Australian liver and cardiothoracic transplant recipients. Am J Trans. 2013;13:1296–1304.
- Na R, Grulich AE, Meagher NS, et al. Comparison of de novo cancer incidence in Australian liver, heart and lung transplant recipients. Am J Trans. 2013;13:174–183.
- Krynitz B, Edgren G, Lindelöf B, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008 - a Swedish population-based study. Int J Cancer. 2013;132:1429–1438.
- Finnish Cancer Registry - Cancer statistics. 2016. [cited 2018 Dec 4]. Available from: https://cancerregistry.fi/statistics/.
- Öhman J, Rexius H, Mjörnstedt L, et al. Oral and lip cancer in solid organ transplant patients – A cohort study from a Swedish Transplant Centre. Oral Oncol. 2015;51:146–150.
- Fernberg P, Edgren G, Adami J, et al. Time trends in risk and risk determinants of non-hodgkin lymphoma in solid organ transplant recipients. Am J Trans. 2011;11:2472–2482.
- Jensen AO, Svaerke C, Farkas D, et al. Skin cancer risk among solid organ recipients: a nationwide cohort study in Denmark. Acta Derm Venereol. 2010;90:474–479.
- Rodriguez Cetina Biefer H, Sündermann SH, Emmert MY, et al. Surviving 20 years after heart transplantation: a success story. Ann Thorac Surg. 2014;97:499–504.
- Roussel JC, Baron O, Périgaud C, et al. Outcome of heart transplants 15 to 20 years ago: graft survival, post-transplant morbidity, and risk factors for mortality. J Heart Lung Trans. 2008;27:486–493.
- Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125–1131.
- Gillison ML, Chaturvedi AK, Anderson WF, et al. Epidemiology of human papillomavirus–positive head and neck squamous cell carcinoma. Jco. 2015;33:3235–3242.
- Sherston SN, Carroll RP, Harden PN, et al. Predictors of cancer risk in the long-term solid-organ transplant recipient. Transplantation. 2014;97:605–611.
- Madeleine MM, Carter JJ, Johnson LG, et al. Risk of squamous cell skin cancer after organ transplant associated with antibodies to cutaneous papillomaviruses, polyomaviruses, and TMC6/8 (EVER1/2) variants. Cancer Med. 2014;3:1440–1447.
- Tufaro AP, Azoury SC, Crompton JG, et al. Rising incidence and aggressive nature of cutaneous malignancies after transplantation: An update on epidemiology, risk factors, management and surveillance. Surg Oncol. 2015;24:345–352.
- Penn I, Hammond W, Brettschneider L, et al. Malignant lymphomas in transplantation patients. Transplant Proc. 1969;1:106–112.
- Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Trans. 2004;4:222–230.
- El-Hamamsy I, Stevens L, Carrier M, et al. Incidence and prognosis of cancer following heart transplantation using RATG induction therapy. Transplant Int. 2005;18:1280–1285.
- Crespo-Leiro MG, Delgado-Jiménez J, López L, et al. The falling incidence of hematologic cancer after heart transplantation. Clin Transplant. 2014;28:1142–1147.
- Kumarasinghe G, Lavee O, Parker A, et al. Post-transplant lymphoproliferative disease in heart and lung transplantation: Defining risk and prognostic factors. J Heart Lung Trans. 2015;34:1406–1414.
