Abstract
Background/purpose: To determine the efficacy and toxicity profile of a stereotactic body radiotherapy (SBRT) boost as a first line treatment in patients with oropharyngeal squamous cell carcinoma (OPSCC).
Materials and methods: We performed a retrospective cohort study in 195 consecutive OPSCC patients with T1-small T3 disease, treated at Erasmus MC between 2009 and 2016 with a SBRT (3 × 5.5 Gy) boost after 46 Gy IMRT. Primary endpoints were disease-specific survival (DSS) and Grade ≥3 toxicity (Common Terminology Criteria). The Kaplan-Meier method and Cox regression model were applied to determine rates and risk factors.
Results: The median follow-up was 4.3 years. Treatment compliance was high (100%). Rates of 5-year DSS and late grade ≥3 toxicity were 85% and 28%, respectively. Five-year overall survival was 67%. The most frequently observed toxicities were mucosal ulceration or soft tissue necrosis (n = 30, 5 year 18%), dysphagia or weight loss (n = 18, 5 year 12%) and osteoradionecrosis (n = 11, 5 year 9%). Current smoker status (hazard ratio [HR] = 2.9, p = .001) and Charlson Comorbidity Index ≥2 (HR = 1.9, p = .03) were was associated with increased toxicity risk. Tooth extraction prior to RT was associated with increased osteoradionecrosis risk (HR = 6.4, p = .006).
Conclusion: We reported on outcomes in the largest patient series to date treated with a hypofractionated boost for OPSCC. Efficacy was good with survival rates comparable to conventionally fractionated (chemo)radiotherapy. Grade ≥3 toxicity profiles showed high rates of soft tissue necrosis and osteoradionecrosis. Strategies to mitigate severe toxicity risks are under investigation to improve the tolerability of the SBRT boost.
Introduction
Stereotactic body radiotherapy (SBRT) allows for precise delivery of ablative radiation doses to the target with improved sparing of surrounding organs at risk [Citation1,Citation2]. SBRT may theoretically be beneficial in the primary treatment of oropharyngeal squamous cell carcinoma (OPSCC), as the oropharynx has critical structures in close proximity. Additionally, SBRT offers greater convenience to patients and radiotherapy departments because of reduced number of fractions. Finally, biological dose escalation may be achieved through SBRT regimens, which theoretically may overcome the intrinsic radioresistance of less radiosensitive disease [Citation3]. Highly hypofractionated regimens, however, may be associated with greater risk of late toxicity, particularly necrotic processes [Citation4–9].
Despite the potential advantages of SBRT for head and neck malignancies and a growing interest internationally [Citation10], there is sparse literature in the setting of newly diagnosed disease. To date, SBRT has been used primarily for re-irradiation [Citation10–12] or rarely, for nasopharyngeal carcinoma [Citation13,Citation14]. The few series on SBRT in the primary setting have either included fewer than 40 patients and diverse head and neck sites [Citation6,Citation15] or median follow-up times less than 18 months [Citation8,Citation16]. In order to evaluate SBRT as a primary treatment modality, studies with long-term follow-up consisting of homogenous patient groups are required.
At our institution, SBRT as a boost following external beam RT has been a standard treatment option for select OPSCC patients since the introduction of a frameless radiosurgery system (Cyberknife; Accuray Inc., Sunnyvale, CA, USA) in 2005. Prior to this, these patients received the boost by brachytherapy. While both techniques deliver a similar highly conformal dose distribution [Citation2], the SBRT boost is advantageous as it is noninvasive and not limited by the strict patient eligibility criteria of brachytherapy or the requirement for specifically trained personnel.
Previously, we reported favorable quality-of-life and toxicity outcomes with SBRT boost up to 24 months post treatment [Citation1,Citation17]. In the current study, we felt it prudent to investigate long-term outcomes especially given the potential for highly hypofractionated RT schedules to increase the risk of late toxicities [Citation4–9].
Material and methods
Patients
Consecutive OPSCC patients treated at the Department of Radiotherapy at Erasmus MC were identified from a prospective radiotherapy planning database which started in 2009. Eligibility criteria for the present study included: treatment with SBRT boost, T1 -“small” T3 (no defined size criterion, but at the discretion of the multidisciplinary tumor board), N0–N2c, M0 primaries. The following exclusion criteria were applied: diagnosis with another primary malignancy within 6 months, previous oropharyngeal cancer or previous head and neck RT. Patients were staged with a CT or MRI for the primary site, ultrasound of the neck, and in the case of N2 disease, thoracic CT.
During the early years of the inclusion period, patients with T1–T2 tumors preferentially received brachytherapy when eligible (n = 58) [Citation17] and the remaining T1–T2, and small T3 tumors, received SBRT boost when eligible (i.e. tumors not adjacent to the thyroid cartilage). Since 2012, patients could receive SBRT boost first line, since our early experience with the SBRT boost regimen was favorable [Citation17]. In total, 195 patients were treated with SBRT boost and fulfilled the in- and exclusion criteria. Patients with poorer performance status (Eastern Cooperative Oncology Group [ECOG] ≥ 2) who were eligible for curative-intent treatment received conventional 70 Gy IMRT. ECOG ≥2 patients may find it challenging to remain still for the 30 min required for delivery of each SBRT fraction, and thus conventional IMRT may be a more suitable treatment option.
Treatment and follow-up
The treatment regimen consists of 46 Gy accelerated IMRT (23 daily fractions, 6 fractions per week) to the primary tumor and neck, followed by a sequential SBRT boost to the primary tumor of 16.5 Gy in 3 daily fractions. The timing is such that total weekly dose during the boost phase never exceeds 16.5 Gy. Thus, the total treatment time for the regimen is approximately 5 weeks. We regard this SBRT boost treatment schedule as a local dose intensification since the calculated biologically effective dose (including reduced treatment time) delivers up to 30.3 Gy (α/β = 10) higher biologically effective dose than a 7-week conventional IMRT regimen for rapidly proliferating tumors [Citation18,Citation19] (equation provided in supporting information). However, transforming this schedule into an equivalent dose in 2-Gy fractions, which does not account for overall treatment time, EQD2 is 67 Gy (α/β = 10). Patients with T3 or N2c disease without contraindication for systemic treatment received two cycles of cisplatin (100 mg/m2) on day 1 and 22 of the IMRT phase. Our early experience with the SBRT boost regimen suggested good outcomes treating patients with N2a-b without chemotherapy, and thus chemotherapy was not given to patients with earlier nodal classification [Citation17]. Patients with positive lymph nodes at the time of diagnosis underwent a neck dissection two weeks following RT, as previously described [Citation20]. The target volume for the accelerated IMRT phase consists of the gross tumor volume (GTV), plus a 1 cm margin on the primary and a 5 mm margin on positive lymph nodes to account for subclinical disease, and an additional 5 mm margin (PTV) to account for set-up error/positional uncertainty. The target coverage objective was PTV V95 > 98%. Following the IMRT phase, a second planning CT scan is obtained. This is rigidly co-registered with the planning CT for the IMRT phase, and the GTV and CTV volumes are transposed. The SBRT PTV consists of the CTV of the primary tumor only, plus a 3 mm margin. The dose is prescribed to the 80% isodose line. The dose constraints for the total plan (EQD2 with α/β = 2) are: spinal cord Dmax <50 Gy and brain stem Dmax <60 Gy (both hard planning constraints); parotid glands Dmean <26 Gy, submandibular glands Dmean <39 Gy, oral cavity Dmean <50 Gy, constrictor muscles Dmean <55 Gy (when achievable). The SBRT boost is delivered on the Cyberknife radiosurgery system [Citation1,Citation17]. Follow-up visits (head-and-neck multi-disciplinary team) were planned every 2 months for the first year, gradually reduced to every 6 months, for a minimum of 5 years.
Endpoints
The primary endpoints were disease specific survival (DSS) and late grade ≥3 toxicity. For DSS, both tumor-related death and toxicity-related death was included as events. Secondary endpoints were overall survival (OS) and locoregional control (LRC). Disease-free survival (DFS) (events: local, regional, distant failure, and death) and progression-free survival (events: local, regional, or distant failure) were also assessed to facilitate comparison of outcomes with the literature.
Toxicity
Acute grade ≥3 dysphagia was scored as requirement for a feeding tube within the first 90 days after RT, according to the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v.4.0). Systematic data on acute dermatitis and mucositis were not available and therefore not scored.
Late toxicity (> 90 days after completion of RT) was scored retrospectively based on CTCAE v.4.0. Of note, CTCAE v.4.0 does not mention hyperbaric oxygen (HBO) for toxicity grading of soft tissue necrosis or osteoradionecrosis (ORN). Version 3 designates HBO as grade 3, as do most recent studies [Citation21–23]; thus, it was scored as grade 3 toxicity for the present study also. In case of recurrent disease, further toxicity scoring was omitted. For patients requiring tube feeding >90 days post-treatment, we evaluated whether this was related to dysphagia (scored as grade 3 dysphagia) or dry mouth (grade 3 xerostomia). Grade 3 trismus was scored as maximal mouth opening <1 cm.
Statistical analysis
Statistical analyses were performed using SPSS software (version 24, IBM Corporation, Armonk, NY). p-Values <.05 were considered statistically significant. The Kaplan-Meier method was used to calculate survival and cumulative incidences of toxicity. OS and DSS were calculated from the first fraction of RT until death from any cause or death from OPSCC, respectively. Patients alive were censored at the date of last follow-up visit. Follow-up time for toxicity endpoints was calculated from the last radiotherapy fraction. Patients were censored from toxicity analysis at time of disease recurrence, death, or last follow-up, whichever came first. Prognostic factors for toxicity were evaluated in univariable Cox regression models, and multivariable models using the forward selection method (entry p < .1, removal p > .1). Covariates assessed included: sex, age (>65 vs. ≤65 years), ECOG performance status (0 vs. 1), smoking (> 10 pack-years vs. ≤ 10, and smoker vs. nonsmoker at diagnosis), Charlson Comorbidity Index score (CCI) (≥2 vs. <2), T stage (T3 vs. T1/T2), N stage (N2 vs. N0–N1), tooth extraction prior to RT, current or previous alcohol abuse, body mass index (BMI) (≤ 22 vs. > 22), disease subsite (tonsil vs other, base of tongue vs other), and bilateral vs unilateral neck RT.
The study protocol was reviewed by the Medical Ethical Committee of the Erasmus Medical Center (EMC17404), and permission was obtained for retrospective anonymized data collection, in accordance with local and national regulations.
Results
Patients
All 195 study patients successfully completed the treatment regimen. Of the 195 study patients, one was lost to follow-up, two died prior to the late toxicity period, and 10 had residual or recurrent disease less than 90 days after RT, leaving 182 (93%) available for late toxicity assessment.
A majority of patients (n = 116, 60%) had stage III-IVA disease according to AJCC 7th edition staging, and 113 (58%) has tonsil primaries. A total of 27 patients had T3 and/or N2c disease (one patient had both). Twelve patients received concurrent chemotherapy, and the additional patients with T3 and/or N2c disease did not (n = 15) due to contraindications to chemotherapy (e.g. comorbidities). A total of 93 patients (48%) had p16 status determined, and among these, 29 (31%) were p16 negative and 64 (69%) were p16 positive. Notably, during the years of study, patients were generally only tested for p16 if they were suspected of having HPV-associated disease (young age, lack of smoking history). For 14 patients p16 status was established retrospectively. The median age was 61 years (range 34–86), and 33 patients (17%) were over the age of 70 years. A total of 103 (53%) were smokers at the time of diagnosis. Additional baseline patient and tumor characteristics are shown in .
Table 1. Baseline characteristics of the study population (n = 195).
Survival
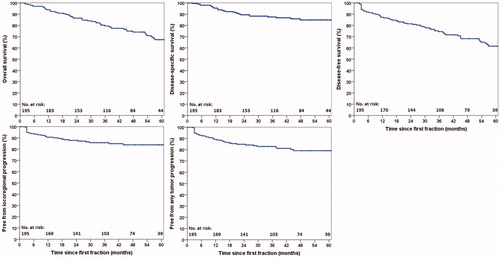
The 2-year and 5-year OS were 87% (2% 1 standard error [SE]) and 67% (4% 1SE), respectively (), while for DFS, these rates were 81% (3% 1SE) and 62% (4% 1SE), respectively. There were 53 deaths (25 OPSCC-related, 12 other malignancy, 2 toxicity-related, 9 other causes, 5 unknown cause). Rates of 2-year and 5-year DSS were 89% (2% 1 SE) and 85% (3% 1SE), respectively (). Median follow-up for surviving patients was 50.6 months (15.0–98.6) and for all patients, 42.8 months (2.1–98.6).
Locoregional control and disease recurrence
The 2-year and 5-year LRC were 88% (2% 1SE) and 84% (3% 1SE), respectively. A total of 37 patients (19%) experienced local, regional, and/or distant disease recurrence. Among the 29 patients (16%) with local and/or regional recurrences, 7 underwent successful salvage surgery. The 5-year local and regional control were 90% and 93%, respectively. A description of disease recurrences and subsequent treatment is provided in supporting information Table S1. A detailed analysis of the location of local and regional recurrences with respect to the radiotherapy fields has previously been published [Citation18].
Acute toxicity
Two patients required a break in treatment due to aspiration/pneumonia, and subsequently completed treatment. During the acute toxicity period, 65 patients (33%) required a feeding tube. One patient had a feeding tube at baseline, and was not included in this assessment.
Late grade ≥3 toxicity
Among the 182 patients available for late toxicity assessment, 47 experienced grade ≥3 late toxicity with an estimated cumulative incidence of 28% (4% 1SE) at 5 years (). Median time to onset of grade ≥3 late toxicity was 10.0 months (3.0–77.6) after RT. The 5-year cumulative incidence of grade ≥3 mucosal ulcers or soft tissue necrosis was 18% (3% 1SE) (). This included one patient with grade 4 toxicity (carotid blow-out which was treated successfully with surgical ligation) and one grade 5 toxicity in a patient who died from tracheal necrosis/bleeding. Among the total 30 patients who experienced grade ≥3 mucosal ulcers or soft tissue necrosis, the time from occurrence until healing was <6 months in 14 patients (47%), 6–12 months in 9 patients (30%), and > 12 months in 6 patients (20%), with one patient (3%) lost to follow-up. The 5-year cumulative incidence of grade ≥3 osteoradionecrosis (ORN) was 9% (3% 1SE) (). Among the total 11 cases of ORN, 5 experienced fracture and/or required surgery (grade 4), and an additional one died from surgical complications (grade 5). The 5-year cumulative incidence of grade ≥3 dysphagia or weight loss was 12% (3% 1SE) (). For comparison with the literature, crude rates of grade ≥3 dysphagia (tube feeding dependence) at 1 and 2 years were 2% (n = 4) and 2% (n = 3), respectively. Additional grade ≥3 toxicities are provided in .
Figure 2. Kaplan-Meier plots showing cumulative incidence of late grade ≥3 toxicity in 182 patients.
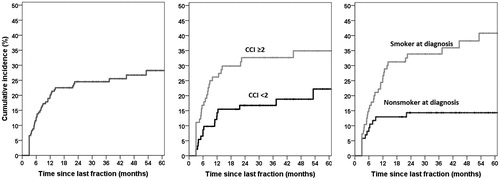
Figure 3. Kaplan-Meier plots showing cumulative incidence of specific late grade ≥3 toxicity in 182 patients.
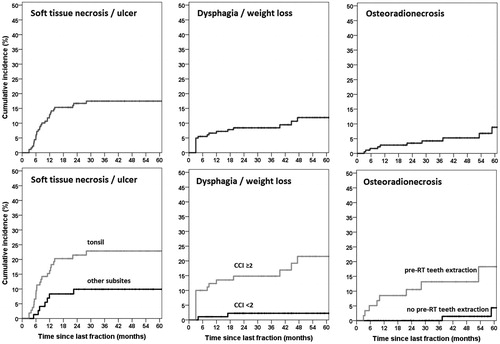
Table 2. Distribution of maximum toxicity scores in 182 evaluated patients (crude numbers).
Prognostic factors for toxicity
Smoking status (smoker at diagnosis) and a CCI ≥2 were associated with higher risk of grade ≥3 late toxicity on both uni- and multivariable analysis (). Current smokers had a 41% (6% 1SE) cumulative 5-year incidence of grade ≥3 late toxicity compared to 14% (4% 1SE) in nonsmokers (p < .01) ().
Table 3. Prognostic factors for grade ≥3 late toxicity.
Tooth extraction prior to RT was predictive for grade ≥3 ORN (HR 6.4, p < .01) (supporting information Table S2 and Figure 2). Only univariate analysis was undertaken for ORN due to the low total number of events. Median time from extraction to start of RT was 18 days, and was not associated with ORN (≤ vs > median time, HR 1.9, p = .4). Smoker at diagnosis and tonsil subsite were significantly associated with increased mucosal ulcers/soft tissue necrosis (p < .05) on multivariable analysis whereas CCI showed a trend towards statistical significance (p < .1) (supporting information Table S2). CCI ≥2 and smoker at diagnosis were associated with severe late dysphagia/weight loss on multivariable analysis (supporting information Table S2).
HPV-related disease
Patients with tumors positive for p16 (n = 63), compared to those with tumors negative for p16 (n = 30), were more likely to have lower CCI scores (p < .01), fewer pack-years (p < .01), nonsmokers (p < .01), higher BMI (p < .01), younger of age (p = .04), and better performance status (p < .01). The cumulative overall grade ≥3 toxicity rate was 15% (5% 1SE) at 5 years in the p16 positive group.
Tumor p16-positive status was strongly associated with lymph node positivity (N1–N2c vs N0) (Spearman’s correlation of 0.45, p < .001). Neck dissection (which was performed for patients with lymph node positivity) was associated with lower risk of grade ≥3 toxicity (hazard ratio = 0.27, p < .001), likely due to the association with p-16 positivity (hence neck dissection was not included in the multivariable toxicity analysis).
Prognostic factors for overall survival
Prognostic factors for overall survival on multivariable analysis (forward model with entry <0.05, removal <0.1) included performance status (ECOG 1 vs 0, HR = 2.6, p < .01), pack years (>10 vs ≤10, HR = 2.2, p = .055), and CCI (CCI ≥2 vs 0–1, HR = 1.9, p = .04). Age, N stage, T stage, current smoking, and tumor subsite did not reach significance. Among the patient subset with known HPV status (n = 93, result of selective HPV testing), the final multivariable model only selects HPV positivity as a prognostic factor (HR = 5.1, p = .001), and all other factors do not reach significance
Discussion
In this single institution series, we report the long-term outcomes of 195 OPSCC patients treated with IMRT plus a SBRT boost, constituting the largest series in the literature of SBRT in the primary setting for head and neck malignancies. We observed a 5-year disease-specific survival and overall survival of 85% and 67%, respectively. Cumulative incidence of Grade ≥3 toxicity at 5 years was 28%. A previous analysis of the SBRT boost regimen at our center reported only 5% late grade ≥3 toxicity. However, this was in a smaller patient cohort (n = 102), with shorter follow-up, and only included T1–T2 tumors [Citation1].
Overall, oncologic outcomes following SBRT boost are similar to those following conventional radiotherapy [Citation23–25]. Few studies with similar patient populations (early T-stage OPSCC, early to advanced nodal disease) are available for meaningful comparison, and rates of HPV-associated disease have not been reported in these studies [Citation24–26]. Nevertheless, one series of early T-stage tumors reported a 5-year OS of 67% [Citation26], identical to the 5-year OS here. Our 2-year DFS of 81% is also consistent with previous studies, which report rates between 82 and 90% [24,26] in T1–T2 tumors with earlier nodal classification (N0–N1) than the present study.
The apparent high rate of p16 positivity among those tested in the present study is partly a reflection of selective p16 testing practices (more often in those lacking a significant smoking/alcohol history). The rate of HPV-associated disease in the Netherlands during the years of study was 40–50% [Citation27,Citation28], although in this population of early T-stage tumors, many with advanced nodal disease, this rate may be higher.
Advantages of the regimen include its tolerability and high compliance rate: all patients completed treatment and only two required short treatment breaks. Conversely, 70 Gy conventional regimens typically require treatment breaks in 10–20% of patients due to acute toxicity, which are associated with worse oncologic outcomes [Citation29,Citation30]. Additionally, the SBRT boost regimen may be a definitive treatment option for patients with advanced disease (stage III–IV) who are not eligible for conventional chemoradiotherapy due to advanced age and comorbidities which preclude concurrent chemotherapy; notably, 33 (17%) of patients in our study were over the age of 70 years. Finally, by avoiding concurrent chemotherapy, ototoxicity, renal dysfunction, and other chemotherapy-related toxicities are avoided.
The major limitation of the SBRT boost regimen is the high rate of severe late toxicity. Notably, 30 patients (16%) developed mucosal ulcers/soft tissue necrosis. Following conventional RT, late mucosal ulceration is relatively rare, occurring in 1–8% of patients [Citation23,Citation24,Citation31,Citation32]. Our 9% rate of ORN is also higher than in the literature (<3% with modern radiation techniques) [Citation5,Citation21,Citation33] with many grade 4 ORN cases which are rare following conventional RT [Citation21,Citation25,Citation33]. The rates of severe late dysphagia we observed (crude rates of 2% at both 1 and 2 years) are slightly lower than those following conventional IMRT in early stage disease (crude 1- and 2-year rates of 7% and 4%, respectively) [Citation34]. ”These findings are consistent with historical data showing increased late toxicity with hypofractionation in head and neck cancers [Citation4,Citation5]. The majority of head and neck SBRT studies in the literature are in the setting of re-irradiation for recurrent disease, and while some of these have reported high incidence (10–17%) of necrotic processes such as soft tissue necrosis and carotid blow-out [Citation7–9,Citation16], others have not found this to be the case, with total late grade ≥3 toxicity rates of 3–6% [Citation11,Citation12]. Different dose fractionation regimens as well as patient selection criteria may largely account for this difference. One other study evaluating SBRT boost (10–25 Gy in 3–5 fractions) following conventionally-fractioned RT (median 50.4 Gy) reported grade ≥3 toxicity in 35% (soft tissue necrosis in 27%) [Citation6].
Risk factors for severe late toxicity were identified, and smoking at the time of diagnosis was the factor which emerged most consistently. This was predictive for total grade ≥3 late toxicity, and also for mucosal ulceration/soft tissue necrosis, and dysphagia/weight loss. The cumulative incidence of severe late toxicity in current smokers was 41%, versus 14% in nonsmokers. This is consistent with the vasoconstrictive and thrombotic microvascular effects of cigarette smoking [Citation35], which likely compound the microvascular injury from radiation which can lead to late toxicity. A less pronounced effect has been demonstrated following conventional RT, where an 18% increase in late grade ≥3 toxicity has been observed in smokers compared to nonsmokers [Citation36].
Tooth extraction prior to RT was strongly associated with ORN. This was despite a median interval of 18 days before the start of treatment, an interval which has been associated with low ORN risk in conventional radiotherapy [Citation37]. Comorbidity as measured by the CCI was significantly associated with general grade ≥3 late toxicity and late grade ≥3 dysphagia/weight loss, and with a trend (p < .1) for increased mucosal ulcers/soft tissue necrosis. Greater comorbidity burden may reduce general physiologic reserve, and increase susceptibility to toxicity. To our knowledge, comorbidity has not previously been examined as a potential prognostic factor for toxicity in head and neck cancer. Finally, tonsil primaries were at higher risk of severe late mucosal ulcers/soft tissue necrosis, potentially due to close proximity to the oropharyngeal wall. It is notable that patients with tumors positive for p16 experienced less grade ≥3 toxicity (5 year 15%), likely because they tend to be nonsmokers, younger, and with fewer comorbidities.
In summary, the regimen of accelerated IMRT and SBRT boost generated good long-term OS and DSS, but with high rates of severe late tissue necrosis, ORN, and overall late grade ≥3 toxicity. To improve the risk-benefit ratio, the protocol for tooth extraction will need evaluation with the goal of reducing rates of osteoradionecrosis. Further study will be needed to determine dose constraints for normal structures, particularly the mandible.
Supplemental Material
Download Zip (91.9 KB)References
- Al-Mamgani A, Van Rooij P, Sewnaik A, et al. Brachytherapy or stereotactic body radiotherapy boost for early-stage oropharyngeal cancer: comparable outcomes of two different approaches. Oral Oncol. 2013;49:1018–1024.
- Teguh DN, Levendag PC, Noever I, et al. Treatment techniques and site considerations regarding dysphagia-related quality of life in cancer of the oropharynx and nasopharynx. Int J Radiat Oncol Biol Phys. 2008;72:1119–1127.
- Baliga S, Kabarriti R, Ohri N, et al. Stereotactic body radiotherapy for recurrent head and neck cancer: a critical review. Head Neck. 2017;39:595–601.
- Maciejewski B, Withers HR, Taylor JM, et al. Dose fractionation and regeneration in radiotherapy for cancer of the oral cavity and oropharynx. Part 2. Normal tissue responses: acute and late effects. Int J Radiat Oncol Biol Phys. 1990;18:101–111.
- Fletcher GH. Hypofractionation: lessons from complications. Radiother Oncol. 1991;20:10–15.
- Lee DS, Kim YS, Cheon JS, et al. Long-term outcome and toxicity of hypofractionated stereotactic body radiotherapy as a boost treatment for head and neck cancer: the importance of boost volume assessment. Radiat Oncol. 2012;7:85.
- Cengiz M, Özyiğit G, Yazici G, et al. Salvage reirradiaton with stereotactic body radiotherapy for locally recurrent head-and-neck tumors. Int J Radiat Oncol Biol Phys. 2011;81:104–109.
- Kodani N, Yamazaki H, Tsubokura T, et al. Stereotactic body radiation therapy for head and neck tumor: disease control and morbidity outcomes. J Radiat Res. 2011;52:24–31.
- Siddiqui F, Patel M, Khan M, et al. Stereotactic body radiation therapy for primary, recurrent, and metastatic tumors in the head-and-neck region . Int J Radiat Oncol Biol Phys. 2009;74:1047–1053.
- Karam I, Yao M, Heron DE, et al. Survey of current practices from the International Stereotactic Body Radiotherapy Consortium (ISBRTC) for head and neck cancers. Future Oncol. 2017;13:603–613.
- Rwigema JC, Heron DE, Ferris RL, et al. The impact of tumor volume and radiotherapy dose on outcome in previously irradiated recurrent squamous cell carcinoma of the head and neck treated with stereotactic body radiation therapy. Am J Clin Oncol. 2011;34:372–379.
- Vargo JA, Ferris RL, Ohr J, et al. A prospective phase 2 trial of reirradiation with stereotactic body radiation therapy plus cetuximab in patients with previously irradiated recurrent squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2015;91:480–488.
- Liu F, Xiao JP, Xu GZ, et al. Fractionated stereotactic radiotherapy for 136 patients with locally residual nasopharyngeal carcinoma. Radiat Oncol. 2013;8:157.
- Hara W, Loo BW, Jr, Goffinet DR, et al. Excellent local control with stereotactic radiotherapy boost after external beam radiotherapy in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2008;71:393–400.
- Yamazaki H, Ogita M, Himei K, et al. Hypofractionated stereotactic radiotherapy using CyberKnife as a boost treatment for head and neck cancer, a multi-institutional survey: impact of planning target volume. Anticancer Res 2014;34:5755–5759.
- Owen D, Iqbal F, Pollock BE, et al. Long-term follow-up of stereotactic radiosurgery for head and neck malignancies. Head Neck. 2015;37:1557–1562.
- Al-Mamgani A, van Rooij P, Tans L, et al. A prospective evaluation of patient-reported quality-of-life after (chemo)radiation for oropharyngeal cancer: which patients are at risk of significant quality-of-life deterioration?. Radiother Oncol. 2013;106:359–363.
- Baker S, Verduijn G, Petit S, et al. Locoregional failures and their relation to radiation fields following stereotactic body radiotherapy boost for oropharyngeal squamous cell carcinoma. Head Neck. 2019. [Epub ahead of print].
- Fowler JF. Correction to Kasibhatla et al. How much radiation is the chemotherapy worth in advanced head and neck cancer? (Int j radiat oncol biol phys 2007;68:1491-1495). Int J. Int J Radiat Oncol Biol Phys. 2008;71:326–329.
- Dronkers EAC, Koljenovic S, Verduijn GM, et al. Nodal response after 46 Gy of intensity-modulated radiotherapy is associated with human papillomavirus-related oropharyngeal carcinoma. Laryngoscope. 2018;128:2333–2340.
- Tsai CJ, Hofstede TM, Sturgis EM, et al. ORN and radiation dose to the mandible in patients with oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2013;85:415–420.
- Ward MC, Ross RB, Koyfman SA, et al. Modern image-guided intensity-modulated radiotherapy for oropharynx cancer and severe late toxic effects: implications for clinical trial design. JAMA Otolaryngol Head Neck Surg. 2016;142:1164–1170.
- Nguyen-Tan PF, Zhang Q, Ang KK, et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity. J Clin Oncol. 2014;32:3858–3866.
- Eisbruch A, Harris J, Garden AS, et al. Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00-22). Int J Radiat Oncol Biol Phys. 2010;76:1333–1338.
- de Almeida JR, Byrd JK, Wu R, et al. A systematic review of transoral robotic surgery and radiotherapy for early oropharynx cancer: a systematic review. Laryngoscope. 2014;124:2096–2102.
- Garden AS, Asper JA, Morrison WH, et al. Is concurrent chemoradiation the treatment of choice for all patients with Stage III or IV head and neck carcinoma? Cancer. 2004;100:1171–1178.
- Melchers LJ, Mastik MF, Samaniego Cameron B, et al. Detection of HPV-associated oropharyngeal tumours in a 16-year cohort: more than meets the eye. Br J Cancer. 2015;112:1349–1357.
- Henneman R, Van Monsjou HS, Verhagen CV, et al. Changes of Human Papillomavirus in Oropharyngeal Squamous Cell Carcinoma and Effects on Survival in the Netherlands Cancer Institute, 1980-2009. Anticancer Res. 2015;35:4015–4022.
- Thomas K, Martin T, Gao A, et al. Interruptions of Head and Neck Radiotherapy Across Insured and Indigent Patient Populations. J Pancreas. 2017;13:e319–e328.
- Russo G, Haddad R, Posner M, et al. Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist. 2008;13:886–898.
- Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35.
- Selek U, Garden AS, Morrison WH, et al. Radiation therapy for early-stage carcinoma of the oropharynx. Int J Radiat Oncol Biol Phys. 2004;59:743–751.
- Nabil S, Samman N. Risk factors for osteoradionecrosis after head and neck radiation: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:54–69.
- Setton J, Lee NY, Riaz N, et al. A multi-institution pooled analysis of gastrostomy tube dependence in patients with oropharyngeal cancer treated with definitive intensity-modulated radiotherapy. Cancer. 2015;121:294–301.
- Silverstein P. Smoking and wound healing. Am J Med. 1992;93(1A):22S–24S.
- Chen AM, Chen LM, Vaughan A, et al. Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int J Radiat Oncol Biol Phys. 2011;79:414–419.
- Koga DH, Salvajoli JV, Alves FA. Dental extractions and radiotherapy in head and neck oncology: review of the literature. Oral Dis. 2008;14:40–44.