Abstract
Background: There is an unmet need for markers predicting the outcome of patients with advanced soft tissue sarcoma (STS) treated with pazopanib. Since toxicity might be related to the anti-tumor activity of the drug, the aim of this study was to determine whether pazopanib-induced proteinuria, hypothyroidism and cardiotoxicity grade 3-4 were associated with outcome.
Methods: The combined results of the EORTC 62043 and 62072 trials were retrospectively assessed and used in a landmark analysis to evaluate the effect of the toxicities on progression-free survival (PFS) and overall survival (OS), using the Kaplan-Meier method and Cox regression models.
Results: Of the 333 eligible patients, 259 patients were included in the analyses, for which a landmark time point of 60 days after randomization/registration was selected. Proteinuria occurred in 25.1%, hypothyroidism in 22.0% and cardiotoxicity grade 3–4 in 5.8% of the patients (any grade in 41.7%). There was no effect of the occurrence of proteinuria (6-months PFS 35.4% for patients with vs. 38.3% for patients without proteinuria, HR 1.01, p = .953), hypothyroidism (41.2% vs. 36.5%, HR 0.82, p = .210) or cardiotoxicity grade 3–4 (26.7% vs. 38.2%, HR 0.97, p = .897) on PFS. Nor was there an effect of proteinuria (6-months OS 63.2% for patients with vs. 74.4% for patients without proteinuria, HR 1.22, p = .196), hypothyroidism (76.2% vs. 70.5%, HR 0.75, p = .093) or cardiotoxicity grade 3–4 (80.0% vs. 77.2%, HR 0.93, p = .801) on OS.
Conclusion: There was no association between the occurrence of pazopanib-induced proteinuria, hypothyroidism and cardiotoxicity and outcome. Therefore, these toxicities cannot be used as predictors for pazopanib activity in patients with advanced STS.
Introduction
Soft tissue sarcomas (STS) are a heterogeneous group of tumors originating from mesenchymal tissue. These rare tumors account for approximately 1% of all adult malignancies and consist of over 50 different histological subtypes [Citation1]. Cornerstone of the treatment of localized STS is surgery, optionally preceded or followed by neoadjuvant/adjuvant therapy, such as radiotherapy, systemic therapy and/or isolated limb perfusion. Unfortunately, a considerable proportion of patients present with locally advanced and/or metastatic STS or will develop these stages over time. For these patients only palliative treatment remains with median overall survival times of 12–18 months [Citation2–7]. First-line treatment usually consists of doxorubicin, sometimes in combination with ifosfamide for fit patients in need of a response [Citation7]. Recently, the addition of olaratumab to doxorubicin as first-line treatment was conditionally approved, based on a randomized phase II study showing a significant improvement in overall survival compared to doxorubicin alone [Citation8].
Up to the last decade, there were not many systemic treatment options for patients failing to doxorubicin-based first-line treatment, but in the past few years several other agents have become available for patients with advanced STS. One of these agents is pazopanib, an oral angiogenesis tyrosine kinase inhibitor (TKI), targeting the vascular endothelial growth factor receptor (VEGFR) and platelet derived growth factor receptor (PDGFR). Based on an improvement in progression-free survival (PFS) and an acceptable toxicity profile, pazopanib was registered as second-line treatment for patients with advanced non-adipocytic STS after failure to prior chemotherapy [Citation9,Citation10].
Although pazopanib yielded an almost three-fold prolongation of PFS over placebo, the observed response rates were low (6–9%) and came at the expense of toxicities [Citation9,Citation10]. On the other hand, there is also a subgroup of STS patients treated with pazopanib with a long-term response (PFS ≥ 6 months, 36%) and long-term survival (OS ≥ 18 months, 34%), including patients remaining progression-free for more than 2 years (3.5%) [Citation11]. These results illustrate the need for markers predicting response and outcome at an early stage.
This unmet need for markers predicting response is underlined by the observation that many patients in daily clinical practice are worried about the effectivity of their treatment, especially in the absence of any side-effects or toxicity. The hypothesis that the occurrence of toxicity is related to the anti-tumor activity of the drug, and that toxicity therefore can be used as a biomarker of efficacy has been tested in multiple combinations of various types of drugs and different types of cancer and for different toxicities. Examples include the occurrence of sunitinib-induced hypertension in renal cell carcinoma (RCC) [Citation12,Citation13] or gastrointestinal stromal tumors (GIST) [Citation14,Citation15], sorafenib-induced diarrhea or hand-foot syndrome in hepatocellular cancer [Citation16, Citation17], pazopanib or sunitinib-induced proteinuria in RCC [Citation18], and VEGF TKI-induced hypothyroidism in RCC [Citation19]. In these studies, the occurrence of VEGF TKI-induced toxicity was associated with an improved response rate and/or survival. Recently, also the occurrence of hematological toxicity in patients with advanced STS treated with the classic chemotherapeutic agent doxorubicin was studied, but no association between the severity of hematological toxicity and response, progression-free survival or overall survival could be demonstrated [Citation20].
For the combination of pazopanib and advanced STS, one of the most common toxicities of pazopanib, hypertension, and its association with outcome has already been studied [Citation21]. In this study, pazopanib-induced hypertension was not associated with outcome in STS patients treated with this agent. However, what does not hold true for hypertension, might be true for other pazopanib-specific toxicities. Therefore, the aim of this study was to investigate whether the occurrence of three other common pazopanib-induced toxicities, namely proteinuria, hypothyroidism and cardiotoxicity, following treatment with pazopanib in patients with advanced STS, was associated with outcome. Additionally, this study provides more insight into the exact incidences of these pazopanib-specific toxicities in patients with advanced STS. These three toxicities were chosen, because they are pazopanib-specific, most likely not to affected/caused by the underlying disease, and well registered during the study period.
Methods
Primary and secondary objectives
The primary objective of this study was to assess the potential association between three pazopanib-induced toxicities and progression-free survival (PFS) of STS patients treated with pazopanib: proteinuria, hypothyroidism and cardiotoxicity related adverse events of grade 3–4. Secondary objectives of this study were to assess the association between these three pazopanib-induced toxicities and overall survival (OS) of STS patients treated with pazopanib, and to describe tumor and patient characteristics of the patients having these toxicities. Additionally, the potential association between cardiotoxicity of any grade and PFS and OS was assessed.
Patient population
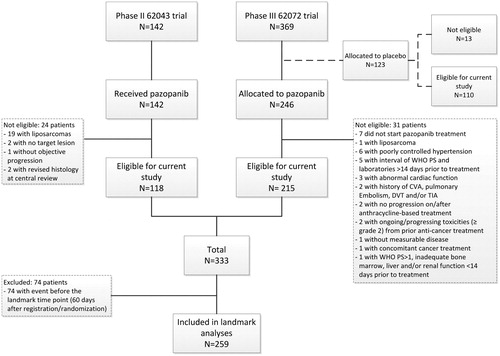
The potential association between these pazopanib-induced toxicities and outcome were assessed retrospectively in the combined results of two prospectively performed studies of the European Organisation for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG): the phase II EORTC 62043 trial [Citation9] and the subsequent phase III EORTC 62072 trial [Citation10]. Details of these studies are listed in supporting information Table S1. All patients eligible for the 62043 trial and 62072 trial who received pazopanib were included, except for patients with liposarcoma. These patients were excluded based on results of the phase II 62043 trial, where pazopanib failed to demonstrate a sufficient beneficial effect in this STS subtype. To investigate the potential impact of treatment with pazopanib, all analyses for the three types of pazopanib-induced toxicities were also performed on the patients in the phase III 62072 trial receiving placebo.
Measurements of toxicity
Proteinuria was measured using the Urine Protein/Creatinine (UPC) ratio and was defined as an UPC ratio greater than 45 mg/mmol, which is equivalent to an albumin/creatinine ratio of greater than 30 mg/mmol. In both studies, urine creatinine and protein levels were reported at baseline, at day 8 of the first treatment period, at day 1 of each following treatment period (28 days) and 28 days after the last treatment administration. A patient was considered to have persistent proteinuria when two of the UPC ratio measurements with an interval of 1–2 weeks minimum exceeded the cutoff point of 45 mg/mmol. Because the time period between two sequential urinary samples was at least 2 weeks in both studies, patients with two consecutive positive tests were categorized as having persistent proteinuria.
To determine the presence of hypothyroidism, thyroid stimulating hormone (TSH) levels were measured and used as a biomarker. Although there is no clear cutoff point, generally an upper normal limit between 2.5 mU/L and 4.0 mU/L is used [Citation22]. For the current study, a patient was considered as having developed hypothyroidism if at least one TSH value surpassed the threshold value of 4.0 mU/L. According to the study protocols, the TSH levels were reported at baseline and at 12 weeks in both studies. Thereafter, levels were reported every 12 weeks (62043 trial) or 16 weeks (62072 trial). Additionally, TSH levels were often measured in between, with peaks around day 8, day 28 and day 56 (coinciding with the times of UPC assessment and start of new treatment periods).
Cardiotoxicity related adverse events were defined as events which occurred after date of randomization/registration and were part of the following list: cardiac ischemia/infarction, edema, hypertension, hypotension, supraventricular arrhythmia or extrasystole, or prolonged QTc interval. Grading of the cardiotoxicity related adverse events was determined according to the National Cancer Institute-Common Terminology Criteria for Adverse Events version 3.0 (NCI-CTCAE v3.0) [Citation23].
Statistical analyses
PFS was defined as time from date of registration/randomization to the first documentation of progression or death, whichever occurred first. If no progression or death was observed, patients were censored at the last date of follow-up. OS was defined as time between date of registration/randomization and date of death. Patients alive at time of clinical cutoff were censored at the date of last follow-up.
To determine the appropriate landmark for further analyses, the cumulative incidence of proteinuria, hypothyroidism and cardiotoxicity related adverse events was assessed. These landmark time points were used to assess the effect of the pazopanib-induced toxicities on PFS and OS compared to patients without toxicity, using the Kaplan Meier method for univariate analyses and Cox regression models for multivariable analyses. The 6-months survival rates are calculated taking the selected landmark as starting point (t = 0) and are reported as percentages with their associated 95% confidence intervals (95% CI). The multivariable models were adjusted for other prognostic factors and included: performance status (0 or 1), gender (male or female), tumor grade (low, intermediate or high), age at time of randomization (≤50 years or >50 years) and histological subtype (leiomyosarcoma, synovial sarcoma or other). In the multivariable model for cardiotoxicity, cardiac history (yes or no) was also included. For the multivariable analyses, an overview summarizing the hazard ratios (HRs) with corresponding 95% CIs for the three pazopanib-induced toxicities is presented. Complete results including all covariates are shown in the supplemental materials (supporting information Table S2–S9). Two-sided tested p-values <.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.4.
Results
Patient population
In total, 333 patients receiving pazopanib (118 out of the 62043 trial and 215 out of the 62072 trial) and 110 patients receiving placebo met the eligibility criteria and were included in the study (). Of the 333 eligible patients receiving pazopanib, 248 patients (74%) developed at least one toxicity, of whom approximately one-third had just one toxicity, 40% a combination of two toxicities and a quarter of the patients all three toxicities. The remaining 85 patients (26%) did not experience any of the three toxicities (supporting information Table S2). Assessment of the incidence of the three toxicities showed that the majority of patients who developed proteinuria, hypothyroidism or cardiotoxicity (both of any grade and of grade 3–4) did so within 60 days after registration/randomization (supporting information Figure S1). Considering this time point as the landmark for further analyses leaves a reasonable number of patients at risk (N = 259).
Association between proteinuria and outcome
Overall, 65 of the 259 patients (25.1%) who received pazopanib and who were included in the landmark analyses had proteinuria at a certain point in time within the landmark period of 60 days, of whom 22 patients had persistent proteinuria (33.8% of the patients with proteinuria, 8.5% of the total study population). These patients more often had a performance score of 1 and a high grade tumor, were more often female and >50 years of age than patients without proteinuria ().
Table 1. Baseline patient characteristics of patients with and without pazopanib-induced proteinuria, hypothyroidism or cardiotoxicity included in the landmark analysis (i.e. patients who did not experience an event, progression or death, before the selected landmark time point of 60 days).
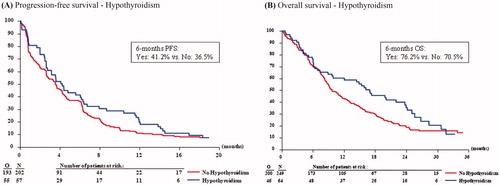
Univariate analysis showed no difference in PFS between patients with proteinuria and patients without proteinuria (), nor for patients with persistent proteinuria (supporting information Figure S2A). After adjustment for other prognostic factors, multivariable analysis also showed no significant prognostic effect of proteinuria or persistent proteinuria on PFS (). The presence of proteinuria or persistent proteinuria also had no significant influence on OS (and ; supporting information Figure S2B).
Figure 2. Kaplan-Meier curves showing the progression-free survival (A) and overall survival (B) of patients with and without pazopanib-induced proteinuria.
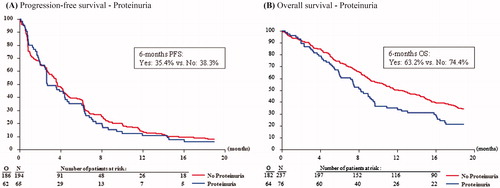
Table 2. Overview of the effect of the three pazopanib-induced toxicities on PFS and OS in patients receiving pazopanib at landmark time point of 60 days, univariate (6-months PFS/OS rates) and multivariable after adjustment for other prognostic factors in multivariable Cox regression analysis. The reported p-values belong to the multivariable Cox regression model. Results of the complete Cox regression analyses are shown in supplemental tables S3–S10.
Association between hypothyroidism and outcome
Approximately one-quarter of the patients developed hypothyroidism within the landmark time point of 60 days (N = 57, 22.0%). These patients were slightly more often female and aged ≤50 years and had slightly more often a performance score of 1 than patients without hypothyroidism ().
There was no effect of the presence of hypothyroidism on PFS (), also not after adjustment for other prognostic factors (). Similarly, there was no significant prognostic effect of the presence of hypothyroidism on OS (), although an trend in favor of patients with hypothyroidism was observed ().
Association between cardiotoxicity related adverse events and outcome
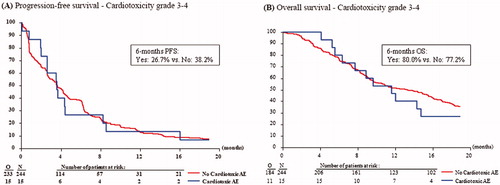
In total, 108 patients (41.7%) experienced a cardiotoxicity related adverse event of any grade within the landmark period of 60 days, of whom 15 patients experienced grade 3–4 cardiotoxicity (13.9% of the patients with any grade cardiotoxicity and 5.8% of the total study population). Three patients experienced arrhythmia, one patient ischemia, but the majority of patients had hypertension (N = 107). Notably, the number of patients experiencing cardiotoxicity other than hypertension was too low to perform separate reliable analyses on. Most of the patients experiencing cardiotoxicity had a performance score of 0, were female, over 50 years old and had intermediate or high-grade tumors. Patients with cardiotoxicity grade 3–4 more often had a cardiac history (46.7%) compared to patients without toxicity (23.0%) and patients with cardiotoxicity of any grade (25.9%) ().
Univariate analysis showed no difference in PFS between patients with and without cardiotoxicity related adverse events grade 3–4 (). Also in multivariable analysis, after adjustment for other prognostic factors, no significant effect of the occurrence of cardiotoxicity related adverse events grade 3–4 on PFS was observed (). Likewise, cardiotoxicity of any grade was not of significant influence (, supporting information Figure S3A). Additionally, there was no association between the occurrence of cardiotoxicity grade 3–4 and OS (), nor in multivariable analysis (). There was also no association between the occurrence of cardiotoxicity of any grade and OS (, supporting information Figure S3B).
Association between toxicity and outcome in patients receiving placebo
For all three pazopanib-induced toxicities, the analyses were also performed on the patients receiving placebo in the 62072 trial. However, no effect on PFS or OS was observed for any of the toxicities in patients receiving placebo (supporting information Tables S5, S7 and S10).
Discussion
The association between three pazopanib-induced toxicities and outcome of patients with advanced STS has been investigated in this study. We observed that pazopanib-induced proteinuria occurred in 25.1% of the patients treated with pazopanib within 60 days after start of treatment, of whom 8.5% had persistent proteinuria. Hypothyroidism was observed in 22.0% of the patients while on treatment with pazopanib. At last, cardiotoxicity (any grade) occurred in 41.7% of patients on treatment, of whom 5.8% had grade 3–4 cardiotoxicity. Additionally, we observed that cardiotoxicity other than hypertension only occurred occasionally in 1.5% of the patients and was only of grade 1 or 2. Overall, no significant prognostic effects were observed for one of these three pazopanib-induced toxicities on PFS or OS. However, although not significant, a trend towards a better prognosis for patients developing hypothyroidism (i.e. high TSH levels) within 60 days after start of pazopanib was observed.
The incidences of the three pazopanib-induced toxicities observed in this study differ slightly from incidences observed in patients with RCC treated with pazopanib. The incidences of proteinuria (25.1%) and hypothyroidism (22.0%) observed in this study were higher than observed for RCC patients. For proteinuria, incidences of 14–18% for patients with RCC have been reported [Citation18,Citation24,Citation25]. Importantly, the majority of these patients underwent a prior nephrectomy, which might have affected the onset of proteinuria. Hypothyroidism was observed in <10%–18% of RCC patients treated with pazopanib [Citation24–26], but to the best of our knowledge, no clear explanation for the differences in incidence of hypothyroidism between patients with RCC and patients with STS exists. On the contrary, the incidence of any grade cardiotoxicity (including hypertension) was slightly lower than observed in RCC patients, varying from 42 to 69%, although definitions of cardiotoxicity differed among RCC studies [Citation24,Citation25,Citation27,Citation28]. Comparable to STS patients treated with pazopanib, the majority of the RCC patients with cardiotoxicity had hypertension, and only a small proportion had myocardial ischemia/infarction or QTc prolongation. Inhibiting the VEGF signaling pathway itself can already induce cardiotoxicity because of its working mechanism [Citation29], but other aspects, such as preceding cancer therapy (anthracyclines), preexisting hypertension or other cardiac history, may also play a role in inducing/worsening cardiotoxicity [Citation30]. Even though most STS patients had received doxorubicin as first-line treatment, which is known for its cumulative cardiotoxicity, the incidence of pazopanib-induced cardiotoxicity was not higher than was described in patients with RCC.
Depending on the severity of toxicity, actions were taken according to protocols. In case of proteinuria, pazopanib was interrupted until the UPC ratio had recovered and pazopanib was restarted at a lower dose. For patients developing hypothyroidism, thyroid replacement therapy was started. At last, in case of cardiotoxicity grade 3–4, pazopanib was discontinued and the cardiotoxicity was treated, whereas for grade 1–2 cardiotoxicity, pazopanib could be continued at the current dose or restarted at a lower dose while treating the cardiotoxicity.
In line with the study on the association between pazopanib-induced hypertension and outcome [Citation21], we did not observe a significant prognostic effect of pazopanib-induced proteinuria, hypothyroidism or cardiotoxicity in patients with advanced STS. This is in contrast to studies examining toxicities induced by anti-VEGF treatment in other types of cancer, such as RCC, hepatocellular cancer, colorectal cancer and GIST. In these studies, a significant association between sunitinib or pazopanib-induced proteinuria and OS was observed in RCC patients [Citation18], and bevacizumab-induced proteinuria was associated with response rate in patients with metastatic colorectal cancer [Citation31]. Additionally, RCC patients with sunitinib-induced or sorafenib-induced hypothyroidism showed increased response rates [Citation32], improved PFS [Citation19,Citation33] and improved OS [Citation19,Citation32]. Furthermore, the occurrence of other sunitinib-induced or sorafenib-induced toxicities, such as hypertension, skin toxicity and diarrhea, was significantly associated with an increase in response rate [Citation12–14], improved PFS [Citation12,Citation14,Citation15] and improved OS [Citation12,Citation14–17,Citation34,Citation35]. To the best of our knowledge, no studies have been published regarding anti-VEGF therapy-induced cardiotoxicity and its association with outcome, except for hypertension.
The thresholds used to determine toxicity, especially for hypothyroidism, and the choice of the landmark time points might be considered as an arbitrary choice to some extent. Whereas the NCI-CTCAE and UPC ratio are well known and established methods to determine and document cardiotoxicity and proteinuria, no clear TSH cutoff point for the diagnosis of hypothyroidism has been agreed upon. Normal ranges of TSH levels may vary by individual, over the day, and even among laboratories, but generally an upper normal limit between 2.5 mU/L and 4.0 mU/L is used [Citation22]. Hence, the TSH threshold value of 4.0 mU/L used in this study is relatively high and conservative, but allowed us to identify each case of hypothyroidism with more certainty, while still a reasonable number of patients were at risk in both groups.
Although the data included in this study were collected prospectively in the two EORTC trials, there are still some potential sources of bias and limitations related to the retrospective design of this study. There were some small differences in, for example, patient and disease characteristics between the two study populations, as well as small differences in the follow-up schedules and in the definition of PFS in the two studies, which might have influenced the outcome. Furthermore, time-to-event bias might have an impact, a type of bias where patients with a favorable response to pazopanib are more likely to continue treatment for a longer period of time, and the longer the exposure to the agent, the higher the chance of developing toxicity. To avoid this potential source of bias, a landmark analysis was used.
To investigate the potential association of the occurrence of toxicity and outcome following treatment with pazopanib, all analyses for the three types of toxicities have been performed on patients receiving the drug as well as on the patients in the phase III trial receiving placebo. As only a few of the patients receiving placebo did not experience progression/death before the landmark time point of 60 days and even a lower number of toxicities was observed in this patient group, unfortunately no real comparison could be made and no clear conclusions on a potential placebo-effect could be drawn.
The hypothesis that the occurrence of toxicity is related to efficacy of the drug and therefore outcome, prevails not only amongst physicians, but also amongst patients. Some patients in daily clinical practice are concerned whether their treatment with pazopanib is effective if they do not experience any side-effects or toxicity. The results of this study may provide reassurance to the treating physicians as well as these patients that the absence of toxicity does not imply that there will be no benefit of treatment with pazopanib.
Conclusion
The occurrence of pazopanib-induced proteinuria, hypothyroidism or cardiotoxicity in patients with advanced STS treated with pazopanib did not have a significant predictive effect and was not associated with outcome. These toxicities can therefore not be used as predictor for pazopanib activity in these patients.
Disclosure of interest
The authors report no conflicts of interest.
Supplemental Material
Download Zip (638.6 KB)Acknowledgments
The authors would like to thank all participants and the investigators of the EORTC 62043 and 62072 trials for their contribution to these studies.
Additional information
Funding
References
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. World Health Organization, International Agency for Research on Cancer. WHO classification of tumours of soft tissue and bone. Lyon: IARC Press; 2013.
- Karavasilis V, Seddon BM, Ashley S, et al. Significant clinical benefit of first-line palliative chemotherapy in advanced soft-tissue sarcoma: retrospective analysis and identification of prognostic factors in 488 patients. Cancer. 2008;112:1585–1591.
- Ryan CW, Merimsky O, Agulnik M, et al. PICASSO III: A phase III, placebo-controlled study of doxorubicin with or without palifosfamide in patients with metastatic soft tissue sarcoma. J Clin Oncol. 2016;1234:3898–3905.
- Tap WD, Papai Z, Van Tine BA, et al. Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft-tissue sarcoma (TH CR-406/SARC021): an international, multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2017;18:1089–1103.
- Seddon B, Strauss SJ, Whelan J, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1397–1410.
- Hensley ML, Miller A, O’Malley DM, et al. Randomized phase III trial of gemcitabine plus docetaxel plus bevacizumab or placebo as first-line treatment for metastatic uterine leiomyosarcoma: an NRG Oncology/Gynecologic Oncology Group study. J Clin Onco. 2015;33:1180–1185.
- Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415–423.
- Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet 2016;388:488–497.
- Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol. 2009;27:3126–3132.
- van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886.
- Kasper B, Sleijfer S, Litiere S, et al. Long-term responders and survivors on pazopanib for advanced soft tissue sarcomas: subanalysis of two European Organisation for Research and Treatment of Cancer (EORTC) clinical trials 62043 and 62072. Ann Oncol. 2014;25:719–724.
- Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103:763–773.
- Rixe O, Billemont B, Izzedine H. Hypertension as a predictive factor of Sunitinib activity. Ann Oncol. 2007;18:1117.
- George S, Reichardt P, Lechner T, et al. Hypertension as a potential biomarker of efficacy in patients with gastrointestinal stromal tumor treated with sunitinib. Ann Oncol. 2012;23(12):3180–3187.
- Rutkowski P, Bylina E, Klimczak A, et al. The outcome and predictive factors of sunitinib therapy in advanced gastrointestinal stromal tumors (GIST) after imatinib failure – one institution study. BMC Cancer. 2012;12:107.
- Howell J, Pinato DJ, Ramaswami R, et al. On-target sorafenib toxicity predicts improved survival in hepatocellular carcinoma: a multi-centre, prospective study. Aliment Pharmacol Ther. 2017;45:1146–1155.
- Cho JY, Paik YH, Lim HY, et al. Clinical parameters predictive of outcomes in sorafenib-treated patients with advanced hepatocellular carcinoma. Liver Int. 2013; 33:950–957.
- Sorich MJ, Rowland A, Kichenadasse G, et al. Risk factors of proteinuria in renal cell carcinoma patients treated with VEGF inhibitors: a secondary analysis of pooled clinical trial data. Br J Cancer. 2016;114:1313–1317.
- Bailey EB, Tantravahi SK, Poole A, et al. Correlation of degree of hypothyroidism with survival outcomes in patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor receptor tyrosine kinase inhibitors. Clin Genitourin Cancer. 2015;13:e131–e137.
- Sleijfer S, Rizzo E, Litiere S, et al. Predictors for doxorubicin-induced hematological toxicity and its association with outcome in advanced soft tissue sarcoma patients; a retrospective analysis of the EORTC-soft tissue and bone sarcoma group database. Acta Oncol. 2018;57:1117–1126.
- Duffaud F, Sleijfer S, Litiere S, et al. Hypertension (HTN) as a potential biomarker of efficacy in pazopanib-treated patients with advanced non-adipocytic soft tissue sarcoma. A retrospective study based on European Organisation for Research and Treatment of Cancer (EORTC) 62043 and 62072 trials. Eur J Cancer. 2015;51:2615–2623.
- Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988–1028.
- Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). National Cancer Institute Division of Cancer Treatment and Diagnosis; 2006 [cited 2018 Jun 6]. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068.
- Matrana MR, Duran C, Shetty A, et al. Outcomes of patients with metastatic clear-cell renal cell carcinoma treated with pazopanib after disease progression with other targeted therapies. Eur J Cancer. 2013;49:3169–3175.
- Hall PS, Harshman LC, Srinivas S, et al. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail. 2013;1:72–78.
- Pinkhas D, Ho T, Smith S. Assessment of pazopanib-related hypertension, cardiac dysfunction and identification of clinical risk factors for their development. Cardiooncology. 2017;3:1–14.
- Maurea N, Coppola C, Piscopo G, et al. Pathophysiology of cardiotoxicity from target therapy and angiogenesis inhibitors. J Cardiovascular Med. 2016;17:e19–e26.
- Touyz RM, Herrmann J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precision Oncol 2018;2:13.
- Feliu J, Salud A, Safont MJ, et al. Correlation of Hypertension and Proteinuria with Outcome in Elderly Bevacizumab-Treated Patients with Metastatic Colorectal Cancer. PLoS One. 2015;10:e0116527.
- Schmidinger M, Vogl UM, Bojic M, et al. Hypothyroidism in patients with renal cell carcinoma: Blessing or curse?. Cancer. 2011;117:534–544.
- Baldazzi V, Tassi R, Lapini A, et al. The impact of sunitinib-induced hypothyroidism on progression-free survival of metastatic renal cancer patients: a prospective single-center study. Urol Oncol. 2012;30:704–710.
- Reig M, Torres F, Rodriguez-Lope C, et al. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J Hepatol. 2014;61:318–324.
- Otsuka T, Eguchi Y, Kawazoe S, et al. Skin toxicities and survival in advanced hepatocellular carcinoma patients treated with sorafenib. Hepatol Res. 2012;42:879–886.