Abstract
Introduction: The aim of this registry-based cohort study was to estimate second cancer (SC) risk following radical prostate cancer (PC) treatment and evaluate if the risk was influenced by radiotherapy.
Materials and methods: We collected data from the Cancer Registry of Norway on all patients with PC as first cancer diagnosis, from 1997 to 2014. Standardized incidence ratios (SIRs) for SC were calculated by comparing our cohort to the standard male population. Subdistribution hazard ratios were estimated in treatment groups, using patients treated with radical prostatectomy (RP) as reference.
Results: We analyzed 24,592 radically treated PC patients. The median follow-up was 7.75 and 6.25 years in the external beam radiotherapy (EBRT) and RP-groups, respectively. SIR for SC was indifferent from the reference population in 24,592 radically treated patients, higher following EBRT, SIR 1.12 (1.07–1.17), and lower following RP, SIR 0.93 (0.87–0.99). EBRT treated patients had higher rectal and urinary bladder cancer incidences, SIR 1.38 (1.16–1.64) and 1.49 (1.31–1.69), respectively. The EBRT patients and the patients treated with radiation after RP (RT after RP) had 38 and 27% higher risk of any SC. We found higher risk of bladder cancer for all treatment groups as compared to RP patients. Only EBRT treated patients showed higher risk of rectal and lung cancer.
Discussion/conclusions: In our study, we found that PC patients treated with EBRT had an increased incidence of SC compared to the general population. Patients treated with EBRT and RT after RP were found to have increased risk of SCs, using RP patients as reference. The risks of rectal and urinary bladder cancer in patients receiving EBRT were higher compared to both the general population and to patients treated with radical prostatectomy. The risk of SC should be taken into account when discussing treatment for patients and designing follow-up.
Introduction
The incidence of second primary cancer is increasing, especially in western countries [Citation1]. In the USA, 18% of all incident malignancies are second cancers (SCs), superseding first cancers of the breast, lung, and prostate [Citation2]. Recently, the CONCORD-3 study showed that cancer survival is increasing worldwide, and Norway is among the countries with the highest survival [Citation3]. For the time period 2012–2016, the 5-year relative survival rate for all cancers in Norway exceeded 70% [Citation4]. Prostate cancer (PC) is the most common cancer among Norwegian males, and about 5000 men (28% of all new male cancers) are diagnosed each year. The incidence rate has tripled since the 1950s, and the 5-year survival is as high as 94% leading to a high prevalence of PC [Citation4]. Even though the mean age at diagnosis is 70 years, a substantial proportion of the patients live long after the diagnosis due to improved treatment as well as a long life expectancy in general (mean 81 years in Norwegian males) [Citation4]. Consequently, the risk of SC in men previously diagnosed with PC cannot be ignored. It is well known that radiotherapy (RT) can cause cancer, but the risk may be altered due to evolving RT techniques and changes in patient demography [Citation5]. Many studies have shown that PC patients having received RT have a higher risk of several cancers as compared to non-irradiated patients. The risk seems to increase with time [Citation6–12]. However, several of the studies showing increased risk of SC included patients from the 1970s and 1980s, and the findings may not be valid for contemporary clinical practice. Other studies have not shown a clear increased risk of cancer after RT for PC, and some authors explain the increased risk with confounding factors such as smoking [Citation13–15]. Consequently, the effect of RT on the incidence of secondary cancers in PC patients is controversial.
The aim of this study was to estimate the risk of SC among Norwegian men with PC given radical treatment as compared to a comparable Norwegian male population and to evaluate if the risk was influenced by RT.
Material and methods
Patients and data collection
The Cancer Registry of Norway (CRN) has registered all new cancer cases in Norway since 1953 [Citation4]. Reporting new cases is mandatory by law, and since 1997, the CRN has also collected treatment data from all RT units in Norway. The completeness of the registry is high (99% coverage of all cancers cases), and the validity is known to be good [Citation16]. Our registry-based cohort study included anonymized CRN data. The study was approved by the CRN regulation and did not require institutional review board approval [Citation17]. We analyzed all patients diagnosed with PC as their first cancer diagnosis, including all patients diagnosed between 1 January 1997 and 31 December 2014, with complete follow-up until 15 June 2018. Date of birth, PC diagnosis, and any SC diagnosis were recorded in all patients. In order to exclude synchronous cancers, SC was defined as any malignancy diagnosed 1 year or more after the PC. Men diagnosed with another cancer prior to the PC were excluded to avoid potential bias caused by RT before 1997. Moreover, the total radiation dose and dose pr. fraction (Gy), date of treatment start, aim of treatment (curative or palliative), region of treatment, the use of external beam radiotherapy (EBRT) vs. high-dose-rate brachytherapy (HDR-BT), and date and type of surgery were registered. Low dose-rate brachytherapy is not used in Norway. Moreover, the CRN dataset did not include data on lymph node irradiation, number of irradiated fields, the use of intensity modulated RT, or volumetric modulated arc therapy (VMAT).
All patients operated on with radical prostatectomy and/or had received RT to the prostate or prostatic region to a total dose of at least 60Gy, were considered radically treated for PC and included in the analyses.
Statistical analysis
We categorized the radically treated PC patients into the following four different groups: radical prostatectomy only (RP), EBRT only, adjuvant or salvage external beam RT after RP (RT after RP), and HDR-BT.
Standardized incidence ratios (SIRs) with 95% confidence intervals (CIs) were calculated by comparing the observed number of SCs in our cohort to the expected number of cancers (except PC) estimated using yearly incidence rates (excluding PC) in 18.5-year age-groups and assuming Poisson distributed counts.
The Aalen-Johansen estimator was used to calculate crude probabilities of SC, treating death from any cause as competing risk [Citation18]. In order to compare treatment groups while adjusting for potential confounding factors, multivariable Fine and Gray competing risk regression was used to estimate subdistribution hazard ratios (SHRs) and corresponding 95% CIs. In addition to type of treatment, the regression model included age at diagnosis (categorized as ≤54, 55–64, 65–74, 75–84, and ≥85 years), tumor stage at diagnosis (localized, regional metastases, and distant metastases), and the time of diagnosis categorized in periods (1997–2003, 2004–2009, and 2010–2014). Since treatment occurred after baseline in some patients, we analyzed treatment as a time varying covariate to avoid immortal time bias. SIR for SC was estimated in the whole cohort and in all radically treated patients. Moreover, SIRs and SHRs for any SC were estimated for all treatment groups. We also analyzed the risk of colon cancer, rectal cancer, urinary bladder cancer, lung cancer, and sarcoma separately. p-Values <.05 were considered statistically significant. Acknowledging the hypothesis-generating nature of our results and to avoid overshadowing by type II errors, we decided not to adjust p-values and CIs for multiple comparisons [Citation19].
All analyses were preformed using the software package STATA IC, version 15 (StataCorp®).
Results
A total of 62,344 patients were registered with PC as their first cancer diagnosis during the inclusion period. We excluded patients with follow-up time less than 1 year (N = 4640), and patients having date for PC diagnosis after the date of radical treatment (N = 10). A total of 57,694 PC patients were eligible for analysis, of which 24,592 were radically treated. Of these, 11,048 patients had received EBRT of at least 60Gy to the prostate, 10,760 had undergone RP, 2162 patients had received RT (60Gy or higher) to the prostatic region after RP, and 622 patients had been treated with HDR-BT. During the study period, the total dose in primary EBRT increased from 70Gy up to 78Gy (2Gy per fraction). Also in adjuvant and salvage RT the total dose escalated from 60Gy given to some patients in 1997 to 70Gy later in the period. HDR-BT for PC was delivered by two high-dose-rate fractions of 10Gy followed by 50Gy conventional EBRT in one center in Norway.
At diagnosis, the patients treated with RP and RT after RP were younger than the patients treated primarily with EBRT or HDR-BT; mean age 62 and 66 years, respectively. Stage at diagnosis also differed between the groups; 58% of the EBRT patients had localized disease (node negative and no metastases) as compared to 68% of the RP patients. The median follow-up time was 7.75 and 6.25 years in the EBRT and RP groups, respectively, whereas the median follow-up time for patients treated with RT after RP and HDR-BT were 8 years ().
Table 1. Patient, tumor, and treatment characteristics.
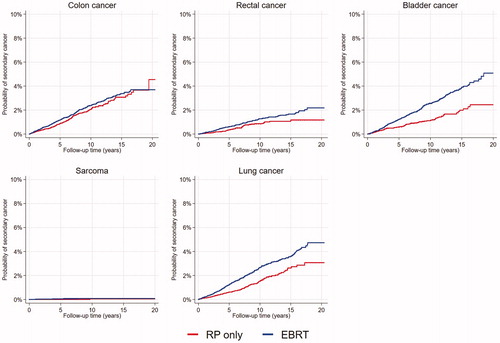
We found 3558 SCs in 24,592 radically treated patients. The most frequent SC was colon cancer, superseding urinary bladder and lung cancer (, available online). The estimated SIR for any SC was 1.02 (95% CI 1.00–1.05) in the whole cohort and 1.04 (95% CI 1.00–1.08) in radically treated patients. As compared to the reference population, SC occurred less frequently in patients treated with RP and more frequently in patients given EBRT (). Urinary bladder- and rectal cancers were more frequent, lung cancers less frequent, and the occurrence of colon cancers and sarcomas similar in radically treated patients. When analyzing SIRs for patients treated with EBRT, we found a higher frequency of rectal cancer and urinary bladder cancer than in the comparable Norwegian male population ().
Table 2. SIRs for all SCs and selected subtypes in 57,694 PC patients.
Multivariable analysis comparing the risk (SHR) of SC in the different treatment groups, showed that patients who received EBRT alone had an almost 40% increased risk and patients treated with RT after RP an almost 30% increased risk of SC, compared to patients treated with RP (). shows the crude risk of SC in the EBRT and RP patients over time.
Figure 1. Risk of SC, all sites, in 21 808 PC patients treated with RP or EBRT. RP: radical prostatectomy; EBRT: external beam radiotherapy.
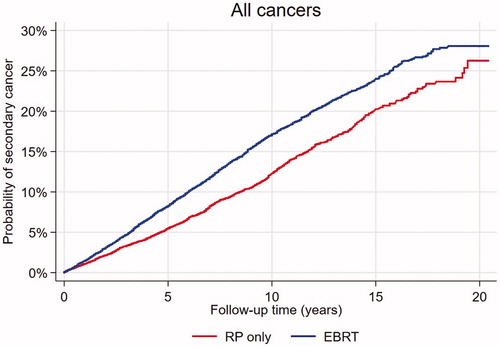
Table 3. Multivariable analysis for the risk of all SC and selected sites in patients treated with EBRT, RT after RP and HDR-BT compared to RP treated patients.
The risk of SC increased with age up to 64 years, was stable from age 65 to 84 and then declined in the oldest patients. Patients with distant metastases at diagnosis had a lower risk of SC than patients with localized and regional disease. Patients diagnosed in the period 2010–2014 had lower risk compared to the other periods (, available online).
We found the risk for rectal cancer, urinary bladder cancer, and lung cancer to be statistically significantly higher in patients treated with EBRT compared to patients treated with RP only (; ). Patients treated with RT after RP and HDR-BT had a statistically significant higher risk of bladder cancer than the RP patients did ().
Discussion
This study based on data from the CRN revealed no difference in SCs incidence between radically treated PC patients and the reference population. The incidence was, however, somewhat higher in patients treated with EBRT and lower following RP, probably reflecting the selection of the healthiest individuals for surgery. In addition, the EBRT- and RT after RP patients had a higher risk of SCs than patients who underwent RP.
The major strength of our study is that it included data from a valid and extensive national cancer registry, with 99% coverage of cases, albeit not with complete registration of all tumor variables. Moreover, the Norwegian public health care system with no private RT units facilitates equal access to cancer care and uniform treatment principles. In this study, the most frequent SCs were colon cancer followed by urinary bladder cancer and lung cancer. In addition to a higher risk of colon cancer in the total cohort, we also found, somewhat surprisingly and not in accordance with previous reports [Citation6,Citation20,Citation21], a higher risk in the RP patients (). The CI was, however, wide and this finding must be interpreted with care.
Since the rectum and urinary bladder inevitably receive high (>50Gy) RT doses in curative EBRT for PC, these organs are at risk of developing radiation induced cancers. In our study, we found that patients who received EBRT had a higher risk of rectal cancer both compared to the general population and to patients treated with RP ( and ). Several previous studies comply with our findings and conclude that RT increases the risk of rectal cancer [Citation8–11,Citation21,Citation22]. Kendal et al. and Hegeman et al. explained an apparently higher rectal cancer risk after RT with unmeasured confounding factors such as tobacco smoking [Citation14,Citation15], which is a well-known risk factor for urinary bladder- and colorectal cancer [Citation23–25]. Although we did not have access to smoking data, we find it reasonable to believe that the non-smokers were selected to prostatectomy more often than smokers. Nevertheless, since our study did not demonstrate an increased incidence of colon cancer in the patients treated with RT, we believe that in-field late RT effects rather than smoking explain the observed higher rectal cancer risk following EBRT. Also corresponding with previous reports, we found a higher risk of urinary bladder cancer in the EBRT patients ( and ) [Citation8,Citation9,Citation15,Citation20–22,Citation26,Citation27]. Patients treated with HRT-BT also had an increased risk of bladder cancer. Given the strong association with tobacco smoking, a higher proportion of smokers probably contributed to these findings. However, the risk of bladder cancer was increased even in patients treated with RT after RP, a group that probably comprises less smokers than those selected to primary EBRT or HDR-BT. It is therefore highly likely that RT was an independent risk factor for urinary bladder cancer in our study population.
RP patients had a 40% lower incidence ratio of lung cancer compared to the general population. The whole PC cohort, all radically treated patients and the RP subgroup, had significantly less lung cancer than the general population. Previous studies support our findings [Citation6,Citation20,Citation21]. Davis et al. reported a SIR for lung cancer in RP patients of 0.68 [Citation21]. When comparing treatment groups, we found that the risk of lung cancer in the EBRT patients was almost twice as high as in the RP patients (). Although several studies report a higher lung cancer risk in patients given EBRT [Citation6,Citation10,Citation28], in a meta-analysis Wallis et al. did not find a higher risk of lung cancer in irradiated patients [Citation9].Studies on patients given curative treatment for other pelvic cancers, also show inconsistent results regarding second lung cancer. In a pooled analysis on patients with rectal or endometrial cancer, Wiltink et al. found no increased risk for any SC following RT [Citation29]. On the other hand, a large SEER (Surveillance, Epidemiology, and End Results Program)-study including almost 80,000 patients undergoing surgery for rectal cancer with or without additional RT, showed an increased lung cancer risk of 17% in the RT group [Citation30]. In our study period as much as 30% of Norwegian males were daily smokers [Citation31], and we find it is reasonable to believe that smoking is a major contributor to the large difference in lung cancer risk found between treatment groups in our study.
Although soft tissue sarcoma may be radiation induced [Citation32], we did not find an increased risk of sarcoma for any treatment groups in our study. However, the number of cases in our cohort was small, and longer follow-up is needed to confirm this finding.
Patients treated with RT after RP had an increased risk of all SCs compared to RP only patients. Still, we did not find increased risk for specific cancers other than bladder cancer. HDR-BT patients were also found to have increased bladder cancer risk. We did, however, not find that brachytherapy influenced on the general risk of SCs. In both groups, the number of patients were relatively low, and firm conclusions would require larger studies with even longer follow-up.
The main limitation of this study is the relatively short median follow-up; 7.75 and 6.25 years for the EBRT and RP patients, respectively. Some authors consider SCs to be radiation induced only if diagnosed after a latency period of 5 years, and some studies only include cancers that occur 5, or even 10, years after RT [Citation8,Citation10–12]. Although several studies have shown that the risk of radiation induced cancer increases even before 5 years [Citation25,Citation33,Citation34], it is likely that more SCs will occur with longer follow-up, and possibly affect the differences between the treatment groups.
According to clinical practice in Norway during the study-period, we assume that the vast majority of the study patients were treated with conformal technique using four or more radiation fields, whereas novel techniques in RT for PC such as VMAT were introduced after the study period. In line with Norwegian national guidelines for PC [Citation35], more patients today receive pelvic lymph node irradiation than in our study period. Both a possible higher SC risk after VMAT [Citation36,Citation37], and pelvic lymph node irradiation, might lead to an even higher SC risk in contemporary patients. Future studies with adequate sample size and follow-up should thus explore the risk of SCs in patients receiving RT with current treatment techniques and principles.
Conclusions
In this large registry-based cohort study with data from the Cancer Registry of Norway, we found that PC patients treated with radical EBRT had an increased incidence of SC compared to the general population. Patients treated with EBRT and RT after RP were found to have increased risk of SCs, using RP patients as reference. The risks of rectal and urinary bladder cancer in patients receiving EBRT were higher both compared to the general population and to patients treated with radical prostatectomy. Patients treated with RT after RP had higher risk of bladder cancer. Although we find no reason to warn against RT in PC treatment, the risk of SC should be taken into account when discussing treatment options for patients and designing guidelines for follow-up.
Supplemental Material
Download Zip (51.4 KB)Disclosure statement
The study has used data from the Cancer Registry of Norway. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Cancer Registry of Norway is intended nor should be inferred.
Additional information
Funding
References
- Wood ME, Vogel V, Ng A. Second malignant neoplasms: assessment and strategies for risk reduction. JCO. 2012;30:3734–3745.
- Travis LB, Demark Wahnefried W, Allan JM, et al. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol. 2013;10:289–301.
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023–1075.
- The Cancer Registry of Norway; [cited 2018 Sept 13]. Available from: https://www.kreftregisteret.no/en
- Ron E. Ionizing radiation and cancer risk: evidence from epidemiology. Radiation Res. 1998;150:S30–S41.
- Brenner DJ, Curtis RE, Hall EJ, et al. Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer 2000;88:398–406.
- Margel D, Baniel J, Wasserberg N, et al. Radiation therapy for prostate cancer increases the risk of subsequent rectal cancer. Ann Surg. 2011;254:947–950.
- Murray L, Henry A, Hoskin P, et al. Second primary cancers after radiation for prostate cancer: a systematic review of the clinical data and impact of treatment technique. Radiotherapy Oncol. 2014;110:213–228.
- Wallis CJ, Mahar AL, Choo R, et al. Second malignancies after radiotherapy for prostate cancer: systematic review and meta-analysis. BMJ 2016;352:i851.
- Moon K, Stukenborg GJ, Keim J, et al. Cancer incidence after localized therapy for prostate cancer. Cancer 2006;107:991–998.
- Baxter NN, Tepper JE, Durham SB, et al. Increased risk of rectal cancer after prostate radiation: a population-based study. Gastroenterology 2005;128:819–824.
- Berrington de Gonzalez A, Curtis RE, Kry SF, et al. Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncol. 2011;12:353–360.
- Zelefsky MJ, Pei X, Teslova T, et al. Secondary cancers after intensity-modulated radiotherapy, brachytherapy and radical prostatectomy for the treatment of prostate cancer: incidence and cause-specific survival outcomes according to the initial treatment intervention. BJU Int. 2012;110:1696–1701.
- Kendal WS, Eapen L, Macrae R, et al. Prostatic irradiation is not associated with any measurable increase in the risk of subsequent rectal cancer. Int J Radiat Oncol Biol Phys. 2006;65:661–668.
- Hegemann NS, Schlesinger-Raab A, Ganswindt U, et al. Risk of second cancer following radiotherapy for prostate cancer: a population-based analysis. Radiat Oncol. 2017;12:2.
- Larsen IK, Smastuen M, Johannesen TB, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45:1218–1231.
- Lovdata. Forskrift om innsamling og behandling av helseopplysninger i Kreftregisteret Lovdata; 2002 [cited 2018 May 4]. Available from: https://lovdata.no/dokument/SF/forskrift/2001-12-21-1477
- Aalen OO, Johansen S. An empirical transition matrix for non-homogeneous markov chains based on censored observations. Scand J Stat. 1978;5:141–150.
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46.
- Braisch U, Meyer M, Radespiel-Tröger M. Risk of subsequent primary cancer among prostate cancer patients in Bavaria, Germany. Eur J Cancer Prevention. 2012;21:552–559.
- Davis EJ, Beebe-Dimmer JL, Yee CL, et al. Risk of second primary tumors in men diagnosed with prostate cancer: a population-based cohort study. Cancer 2014;120:2735–2741.
- Bhojani N, Capitanio U, Suardi N, et al. The rate of secondary malignancies after radical prostatectomy versus external beam radiation therapy for localized prostate cancer: a population-based study on 17,845 patients. Int J Radiat Oncol. 2010;76:342–348.
- Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. JAMA 2008;300:2765–2778.
- Leppert JT, Shvarts O, Kawaoka K, et al. Prevention of bladder cancer: a review. Eur Urol. 2006;49:226–234.
- Boorjian S, Cowan JE, Konety BR, et al. Bladder cancer incidence and risk factors in men with prostate cancer: results from Cancer of the Prostate Strategic Urologic Research Endeavor. J Urol. 2007;177:883–887; discussion 887–888.
- Van Hemelrijck M, Feller A, Garmo H, et al. Incidence of second malignancies for prostate cancer. PLoS One. 2014;9:e102596.
- Neugut AI, Ahsan H, Robinson E, et al. Bladder carcinoma and other second malignancies after radiotherapy for prostate carcinoma. Cancer 1997;79:1600–1604.
- Nam RK, Cheung P, Herschorn S, et al. Incidence of complications other than urinary incontinence or erectile dysfunction after radical prostatectomy or radiotherapy for prostate cancer: a population-based cohort study. Lancet Oncol. 2014;15:223–231.
- Wiltink LM, Nout RA, Fiocco M, et al. No increased risk of second cancer after radiotherapy in patients treated for rectal or endometrial cancer in the randomized TME, PORTEC-1, and PORTEC-2 trials. J Clin Oncol. 2015;33:1640–1646.
- Warschkow R, Guller U, Cerny T, et al. Secondary malignancies after rectal cancer resection with and without radiation therapy: a propensity-adjusted, population-based SEER analysis. Radiotherapy Oncol. 2017;123:139–146.
- Statistics Norway. Statistisk sentralbyrå. 2018; [cited 2018 September 13]. Available from: https://www.ssb.no/en
- Riad S, Biau D, Holt GE, et al. The clinical and functional outcome for patients with radiation-induced soft tissue sarcoma. Cancer 2012;118:2682–2692.
- Nieder AM, Porter MP, Soloway MS. Radiation therapy for prostate cancer increases subsequent risk of bladder and rectal cancer: a population based cohort study. J. Urol. 2008;180:2005–2009; discussion 2009–2010.
- Abdel-Wahab M, Reis IM, Hamilton K. Second primary cancer after radiotherapy for prostate cancer—a seer analysis of brachytherapy versus external beam radiotherapy. Int J Radiat Oncol, Biol, Phys. 2008;72:58–68.
- Helsedirektoratet. Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av prostatakreft [Internet]. 2015 [cited 2017 May 30]. Available from: http://www.helsebiblioteket.no/retningslinjer/prostatakreft
- Murray LJ, Thompson CM, Lilley J, et al. Radiation-induced second primary cancer risks from modern external beam radiotherapy for early prostate cancer: impact of stereotactic ablative radiotherapy (SABR), volumetric modulated arc therapy (VMAT) and flattening filter free (FFF) radiotherapy. Phys Med Biol. 2015;60:1237–1257.
- Stokkevag CH, Engeseth GM, Hysing LB, et al. Risk of radiation-induced secondary rectal and bladder cancer following radiotherapy of prostate cancer. Acta Oncol. 2015;54:1317–1325.