Abstract
Background: For a few types of cancer, lower socioeconomic status (SES) is associated with higher incidence, and for even more cancer types it is associated with having a less favorable tumor stage at diagnosis. For endometrial cancer (EC), however, there is no clear evidence of such associations with SES. There is a need for analysis of sociodemographic disparities in EC incidences according to stage at diagnosis, which may provide support for trying to improve early detection of EC.
Material and methods: Stage-specific incidences of endometrioid and non-endometrioid endometrial carcinomas [EECs (∼90% of all EC cases) and NECs (∼10%)] were analyzed for the population of the Western Swedish Healthcare Region, taking into account year (1995–2016), age, educational level (low, intermediate and high), and immigrant status (Swedish-born, foreign-born). All EC cases were identified and data were obtained from population-based registries.
Results: Stage distribution of diagnosed EECs differed significantly according to the educational level of patients who were aged between 50 and 74 years at diagnosis, but not in the case of younger or older patients. An analysis based on 3113 EEC cases aged 50–74 years at diagnosis revealed marked disparities in the stage-II to stage-IV EEC incidences but not in the stage-I EEC incidence. Compared to women with a high level of education, the incidence rate ratios of stage-I, stage-II and stage-III and -IV EEC in women with a low level of education were 1.00 (95% CI: 0.90–1.12), 1.65 (1.13–2.42), and 1.82 (1.33–2.49), respectively. For NEC, we found no such association.
Conclusions: Elevated incidences of stage-II to stage-IV EEC in 50- to 74-year-old women with a low level of education suggest that there should be targeted health service trials aimed at improving awareness of EC. Well-targeted EC awareness programs might lead to considerable health benefits.
Introduction
Globally, endometrial cancer (EC) is the sixth most common malignant disorder in women [Citation1]. Age-standardized incidence rates are highest in Europe and North America and lowest in developing countries. For example, in the age range 30–84 years, an incidence rate of 40 per 100,000 has been reported for Sweden and 10 per 100,000 for South Africa [Citation2,Citation3]. Obesity is an established risk factor. It has been estimated that a 5 kg/m2 (which corresponds to a weight gain of 13 kg in women with a normal BMI) increase in body mass index (BMI) is associated with an increased risk of EC [Citation4] and about 90% of type-1 EC patients are obese [Citation5]. Other workers have reported that one-third of all EC cases can be attributed to high BMI [Citation6]. Adherence to guidelines for cancer prevention, including maintenance of a healthy weight, a physically active lifestyle, and a healthy diet with more plant-based food and limited alcohol consumption, has been reported to be associated with a reduction in the risk of EC to 23–60% [Citation7].
Obesity is usually more prevalent in groups with low SES [Citation8]. However, there is no clear evidence that having a lower socioeconomic status (SES) is associated with having a higher incidence of EC. Reports from Denmark (data from the period 1994–2003) and Finland (1971–1995) have indicated that there are higher incidence rates of EC in groups with higher SES [Citation9,Citation10], although such gaps in the incidence of SES between groups with different types of SES were found to disappear in Finland in the early 1980s. Swedish data for the period 1997–2008 show similar incidence rates of EC in population groups with different levels of education [Citation3].
For EC, there is evidence that low SES is associated with mortality after diagnosis. Low SES has been reported to be associated with worse survival rates following diagnosis of EC [Citation3,Citation9,Citation11]. Different ethnic groups may be associated with more or less favorable prognosis. Also, the incidence of EC may differ between ethnic groups. Data from the USA show that white women have the highest incidence of EC, but twice as high mortality after diagnosis among black women, who tend to have less favorable histology, grade, and stage when diagnosed [Citation12].
Stage is the main prognostic factor for EC. Generally, the incidence of endometrioid endometrial carcinoma (EEC) is much higher than the incidence of non-endometrioid endometrial carcinoma (NEC), but the proportion of stage-II to stage-IV tumors is higher for NEC. There have been no analyses of sociodemographic disparities in incidence of EC taking into account the stage at diagnosis. In this study, we attempted to identify population groups with elevated incidence rates of EC at stages II–IV EC. More specifically, we wanted to determine whether disparities in stage-specific incidences of EEC and NEC might be associated with population groups defined by age, educational level (low, intermediate, and high) and immigrant status (Swedish-born, foreign-born), using data from the population of the Western Swedish Healthcare Region (WSHCR).
Materials and methods
Study population
The study population comprised women living in the WSHCR, which has two million inhabitants, corresponding to 20% of the Swedish population.
Patient data
The patients who were eligible for this study were all women who resided in the study area and had an EC diagnosed between 1 January 1995 and 31 December 2016. We were able to identify all the EC patients by considering all notifications of EC in the obligatory Swedish Cancer Registry [Citation13]. Provided informed consent had been obtained from a patient, clinical data were recorded in the WSHCR Clinical Register for Endometrial Cancer, with 99% coverage (period 1995–2006) [Citation14], and the national Swedish Endometrial Cancer Quality Registry, with 95% coverage (period 2006–2016) [Citation15]. Histopathological type, surgical stage, and clinical stage were reported by the gynecologist who performed the surgery. Histopathological subtype was classified as EEC, including mucinous adenocarcinoma, or NEC, including carcinosarcoma, clear cell adenocarcinoma, and serous adenocarcinoma. Patients were staged according to the International Federation of Gynecology and Obstetrics (FIGO) guidelines from 2009 [Citation16]. Data on surgical stage were used primarily and clinical stage secondarily. Patient data were linked to the Statistics Sweden population registers, using the unique Swedish identity numbers [Citation17,Citation18]. Data on country of birth and educational level could then be obtained and patients were classified as being Swedish or foreign-born, referred to as immigration status, and according to their level of education, which was derived from the number of school years completed at the end of the year of diagnosis [“low”: ≤9 years (primary school level); “intermediate”: 10–12 years (high school/pre-university level); and “high”: ≥13 years (university level)].
Population data
Statistics Sweden provided the population data for incidence calculations, i.e., female population size by calendar year and five-year age groups. We obtained data that permitted additional population stratification according to level of education in the age range 30–74 years. The lower age limit, 30 years, was considered appropriate for judgment of previous educational achievements. In fact, only two of the EC patients were below 30 years of age at diagnosis. The upper limit, 74 years, was used due to availability of registry data. We also obtained population data that permitted stratification of the population according to immigration status, besides year (though, restricted to the period 2000–2016) and age.
Statistical methods
Poisson regression [Citation19] was used to estimate total and stage-specific incidence rates of EEC and NEC, respectively, incorporating the following covariates: year of diagnosis, age at diagnosis, and educational level or immigration status. Absolute incidence rates were estimated by model-based marginal means. The analyses were carried out using IBM Statistics for Windows version 25.0 (IBM Corp., Armonk, NY, USA).
Results
Numbers of patients
Of the 5833 patients who were identified, we were able to classify 5579 according to histopathological subtype and stage (4921 EEC patients and 658 NEC patients; ). For these patients, the ages at diagnosis were distributed as follows: EEC, 172 (4%) aged 30–49 years, 3149 (64%) aged 50–74 years, and 1599 (33%) aged 75 years or more; NEC, 15 (2%) aged 30–49, 361 (55%) aged 50–74, and 282 (43%) aged 75 or more.
Figure 1. Numbers of patients identified and analyzed, stratified by histopathologic subtype (endometrioid endometrial carcinoma, EEC; non-endometrioid endometrial carcinoma, NEC) and age at diagnosis.
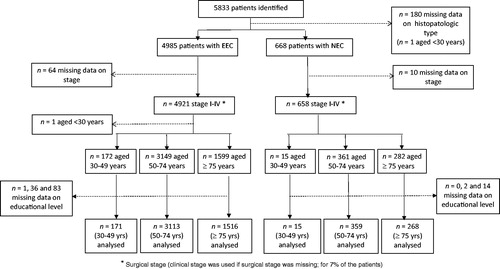
Of the 5833 patients identified, 652 (11%) were not born in Sweden and of these, 351 (6%) were born outside the Nordic countries.
Age-specific incidence rates
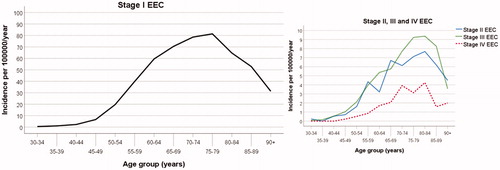
The incidence rate of stage-I EEC started to increase markedly from the age range 45–49 years up to the age range 75–79 years, and the stage-II, -III and -IV EEC rates continued to increase up to 80–84 years (). The incidence rate of stage-I–IV NEC showed a similar age pattern (Figure S1). We found no statistical evidence that year of diagnosis had any influence on the incidence rate of EEC (p = .12). Year of diagnosis showed a significant association with the incidence rate of NEC (p < .001). However, no systematic time trend in the incidence of NEC was apparent (Figure S1). We also found that the estimated age-specific incidence pattern for NEC was not significantly affected by incorporating year of diagnosis in the Poisson regression model.
Figure 2. Age-specific incidence rates of endometrioid endometrial carcinoma (EEC) according to stage at diagnosis for the female population of the Western Swedish Health Care Region (1995–2016). The stage-II, stage-III, and stage-IV incidence rates are presented in a separate graph with a magnified scale on the y-axis.
Stage at diagnosis and level of education
Taking into account the level of education of the patient, the distributions of stages of EEC, stratified by age at diagnosis (30–49, 50–74, and 75+), are given in . Having a lower educational level was significantly associated with a higher proportion of stage-II to stage-IV EECs in the patients who were between 50 and 74 years old at diagnosis (p = .009; chi-squared test). In patients who were younger or older at diagnosis, no such socioeconomic gradient was evident [patients aged 30–49 years, p = .11; patients aged 75 or more, p = .85]. We also assessed the stage distribution according to level of education in the age groups 50–59 and 60–74 years, bearing in mind that EEC in peri-menopausal women is often more characterized by unspecific presenting symptoms (irregular bleeding) than in post-menopausal women. The proportions of stage-II to stage-IV EECs in the groups with different levels of education differed less markedly in the 50- to 59-year age group (low educational level, 21.0% stage-II to stage-IV EECs; intermediate educational level, 20.2%; high educational level, 15.6%), than in the 60- to 74-year age group (low, 20.5%; intermediate, 16.3%; high; 12.8%).
Table 1. EEC stratified by age at diagnosis and educational level and stage distribution.
For NEC, there was no significant difference in the stage distribution in the different patient groups with different levels of education (p = .68; Table S1).
We found statistically valid associations with educational level for the stage-II and stage-III to stage-IV EEC incidence rates but not for the stage-I EEC rate in women aged between 50 and 74 years (). Compared to women with a high level of education, the estimated incidence rate ratios (IRRs) of stage-I, stage-II and stage-III to stage-IV EEC in women with a low level of education were 1.00 (95% CI: 0.90–1.12), 1.65 (1.13–2.42), and 1.82 (1.33–2.49), respectively. The corresponding IRRs in 50- to 74-year-old women with an intermediate level of education were 0.93 (0.84–1.04), 1.05 (0.71–1.55), and 1.38 (1.01–1.87). We found no statistical evidence that these stage-specific incidence associations with education level were modified by age or calendar year [p-values for the interaction terms education × age (with age classified as 50–54, 55–59, 60–64, 65–69, and 70–74 years) and education × year were > .3 when each of these terms was added to the Poisson regression model].
Figure 3. Stage-specific incidence rates of endometrioid endometrial carcinoma (EEC) for the 50- to 74-year-old female population of the Western Swedish Healthcare Region (1995–2016). Estimates of the incidence rates with 95% CIs are shown for each population group according to the women’s level of education (low, medium, high). The p-values were obtained by testing the null hypothesis of no difference in incidence rates in the population groups with different levels of education.
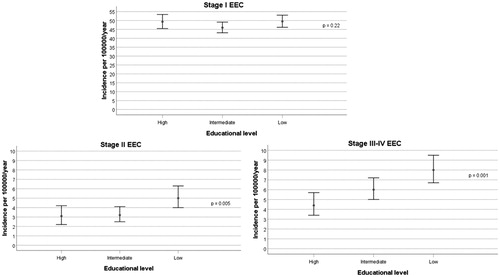
Given that most (82%) of the 50- to 74-year-old EEC patients were diagnosed at stage-I (), we accordingly found that the total incidence of EEC was modestly influenced by level of education: as compared to women with a high level of education, the IRRs for women with low and intermediate educational attainments were 1.11 (1.01–1.22) and 0.98 (0.98–1.18), respectively.
Stage at diagnosis and immigration status
The proportion with low level of education was higher in the foreign-born patients aged 50–74 years at diagnosis than in their Swedish-born counterparts (46.6% vs. 38.1%). However, we found no indication of a incidence rate of stage-II to stage-IV EEC in foreign-born women aged 50–74 years, in the period 2000–2016 [IRR compared to Swedish-born: 0.91 (95% CI: 0.69–1.20)]. The corresponding IRR for stage-I EEC was estimated to be 0.91 (0.80–1.04).
Discussion
In this population-based study based on the Western Swedish Healthcare Region, involving 5579 women diagnosed with EC in the period 1995–2016, we found that having a lower level of education was associated with markedly higher incidence rates of stage II to stage-IV EEC in 50- to 74-year-old women. Foreign-born women did not have a higher incidence rate of late-stage EEC, despite the fact that a higher proportion of the immigrants had a low level of education according to registry data.
In our analyses, less than 7% of the patients identified were excluded because of missing data on histopathological type, stage, or educational level. Misclassification of histopathology was reduced by applying reference demonstrations by two pathologists. Age, which we considered to be a potential confounder (older age was postulated to be associated with lower educational level and more advanced stages), was controlled for by a variable categorized by 5-year intervals.
Educational level is an important indicator of SES, and an essential factor affecting life circumstances and health. Educational attainment, which is usually completed before the age of 30, is associated with occupation, income, residence area, major risk factors for adverse health outcomes, health literacy, and so on. Several studies have shown a concentration of poor health-related behavior, such as smoking, high alcohol consumption, and lack of physical activity, in groups with lower SES [Citation20,Citation21]. Socially deprived population groups may have higher burdens of risk factors for EEC, such as obesity, diabetes, and physical inactivity [Citation22]. Nevertheless, our data did not provide convincing support for a general association between educational level and EC incidence; so the present results are in accordance with previous results [Citation9,Citation23]. On the other hand, our new findings concern SES-related disparities in the incidences of stage-II to stage-IV EEC, but not in the incidence of stage-I EEC in 50- to 74-year-old women (observed in the period 1995–2016). We found no such associations for NEC, which had a much lower number of cases and therefore lower statistical power. We also found that NEC is less dependent on hyperoestrogenic risk factors, e.g., obesity, which are more prevalent in women with a lower level of education [Citation24]. We believe that the new result regarding EEC is due to varying knowledge about symptoms, and behaviors following symptoms, between different patient groups, possibly related also to inadequate interactions with healthcare. The cardinal early symptom of EC is irregular vaginal bleeding. The association between having a lower level of education and higher incidence of stage-II to stage-IV EEC was only evident in the age group 50- to 74-year age group. EC in younger, pre-menopausal women is more often characterized by unspecific presenting symptoms. Younger patients are less likely to have fast-track referrals than elderly women [Citation25]. The result in question concerns peri-menopausal and post-menopausal women, and may be a consequence of more frequent delay in contacting the general practitioner or gynecologist when irregular vaginal bleeding occurs in those with a low level of education. Thus, our findings could be due to longer patient’s delay in this group. Doctor’s delay may also have played a role, if interaction between women with symptoms, but with a low level of education, and healthcare personnel functions less well, delaying a correct investigation of bleeding disorders. However, it is unclear to us why a true effect of SES-related differences in doctor’s delay on the stage distribution at diagnosis, if present, would appear only for the 50- to 74-year-old patients. Younger patients are less likely to have fast-track referrals since there are often no alarm symptoms. It is notable that the SES gradient in the proportions with stage-II to stage-IV ECCs at diagnosis was less pronounced for 50- to 59-year-old patients than for 60- to 74-year- old patients (the younger patients more often have unspecific presenting symptoms). When women become older than 75 years, a general change in the healthcare situation, with more contact with healthcare professionals, may lead to a moderation of SES-related differences in delayed diagnosis.
Immigration status was not associated with a higher incidence rate of advance-stage EEC, despite there being a higher proportion with a low level of education among the foreign-born women aged 50–74 years. We acknowledge the following limitations of this particular analysis: (i) the group of foreign-born women was small and ethnically heterogeneous, and (ii) using registered information on the educational level of foreign-born individuals might imply more misclassification and therefore accentuated information bias [Citation26]. The latter limitation led us to not adjust for educational level when estimating incidence associations with immigrant status.
Awareness programs can lead to improved knowledge about symptoms of EC [Citation27], and well-targeted awareness programs might possibly improve early detection of EC. Coordination of awareness and screening programs may be attractive for cancer types with elevated late-stage incidence rates in similar population groups. We have performed an analysis of CRC incidence rates within the WSHCR and have found marked socioeconomic gradients in the stage-II to stage-IV rates, but not the stage-I rates, in Swedish-born women aged between 55 and 74 years [Citation28]. Organized screening for bowel cancer is expected to be implemented in the WSHCR in 2019 (in Sweden, bowel cancer screening was partly implemented in 2008, but only in the Stockholm-Gotland Healthcare Region). The starting age for bowel cancer screening is generally between 50 and 60 years, and the screening usually ends at the age of 75. Attendance in bowel cancer screening is considerably lower in socially deprived population groups [Citation29]. Thus, considering (i) our present findings, which show elevated incidence rates of stage-II to stage-IV EECs in 50- to 74-year-old women with a low educational level (this SES gradient was even more pronounced in 60- to 74-year-old women), and (ii) the expected challenges of bowel cancer screening, we recommend the setting up of a coordinated, targeted health service trial aimed at improving awareness of EC and bowel cancer screening attendance. An awareness program should be designed to counteract both patient’s delay and doctor’s delay. We encourage such randomized health service trials, designed to evaluate new strategies in a randomized fashion [Citation30,Citation31]. Implementation of EC awareness programs into existing programs for bowel cancer screening appears to be an effective option. Strategies for reducing the socioeconomic gradient in the outcomes of such coordinated programs are important. Based on cluster-randomized trials on such strategies for bowel cancer screening attendance, it has been concluded that achievements through written materials alone will be challenging [Citation32].
The health benefits of improved early detection of EC may be considerable. In Sweden, for example, ∼850 women between 50 and 74 years age are diagnosed with EC each year, and ∼150 of these women have stage-II to stage-IV EC. The average excess mortality is ∼10 times higher for stage-II to stage-IV EC than for stage-I EC. If improved early detection changes the distribution of stage I/stage-II to stage-IV EC from 700/150 to, say, 750/100, an average reduction in excess mortality of ∼20% might be achieved for EC patients aged 50–74 years at diagnosis.
Ethical approval
The study was approved by the Regional Ethical Review Board in Gothenburg, Sweden.
Supplemental Material
Download Zip (144.9 KB)Acknowledgments
We thank Mr. Anders Holmén for work with sociodemographic data. This article was finalized during the last author’s sabbatical at the School of Public Health, Imperial College, London, which was supported by the Wenner-Gren Foundations.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386.
- World Health Organization, International Agency for Research on Cancer. Global Cancer Observatory. Cancer Today; 2018 [cited 2018]. Available from: https://gco.iarc.fr
- Socialstyrelsen. Cancer i Sverige. Stockholm: Socialstyrelsen; 2011.
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–578.
- Fader AN, Arriba LN, Frasure HE, et al. Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol. 2009;114:121–127.
- Pearson-Stuttard J, Zhou B, Kontis V, et al. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol. 2018;6:e6–e15.
- Kohler LN, Garcia DO, Harris RB, et al. Adherence to diet and physical activity cancer prevention guidelines and cancer outcomes: a systematic review. Cancer Epidemiol Biomarkers Prev. 2016;25:1018–1028.
- Mackenbach JP, Stirbu I, Roskam AJ, et al. Socioeconomic inequalities in health in 22 European countries. N Engl J Med. 2008;5;358:2468–2481.
- Jensen KE, Hannibal CG, Nielsen A, et al. Social inequality and incidence of and survival from cancer of the female genital organs in a population-based study in Denmark, 1994–2003. Eur J Cancer. 2008;44:2003–2017.
- Pukkala E, Weiderpass E. Time trends in socio-economic differences in incidence rates of cancers of the breast and female genital organs (Finland, 1971–1995). Int J Cancer. 1999;81:56–61.
- Von Behren J, Abrahao R, Goldberg D, et al. The influence of neighborhood socioeconomic status and ethnic enclave on endometrial cancer mortality among Hispanics and Asian Americans/Pacific Islanders in California. Cancer Causes Control. 2018;29:875–881.
- Chatterjee S, Gupta D, Caputo TA, et al. Disparities in gynecological malignancies. Front Oncol. 2016;6.
- Cancerregistret: Socialstyrelsen; 2019. Available from: https://www.socialstyrelsen.se/register/halsodataregister/cancerregistret
- Svanvik T, Sundfeldt K, Stromberg U, et al. Population-based cohort study of the effect of endometrial cancer classification and treatment criteria on long-term survival. Int J Gynaecol Obstet. 2017.
- Livmoderkroppscancer: Regionalt Cancercentrum Väst; 2019. Available from: https://www.cancercentrum.se/vast/cancerdiagnoser/gynekologi/livmoderkropp/
- Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105:109.
- Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–667.
- Folkbokföringen: Statistics Sweden; [cited 2016 Dec 12]; 2019. Available from: https://www.scb.se/sv_/Hitta-statistik/Artiklar/Folkbokforingen—Ett-register-i-standig-forandring/
- Clayton D, Hills M, Statistical models in epidemiology. Oxford: Oxford University Press; 1993.
- Laaksonen M, Prattala R, Lahelma E. Sociodemographic determinants of multiple unhealthy behaviours. Scand J Public Health. 2003;31:37–43.
- Laaksonen M, Prattala R, Helasoja V, et al. Income and health behaviours. Evidence from monitoring surveys among Finnish adults. J Epidemiol Community Health. 2003;57:711–717.
- Amant F, Moerman P, Neven P, et al. Endometrial cancer. Lancet 2005;366:491–505.
- Dalton SO, Schuz J, Engholm G, et al. Social inequality in incidence of and survival from cancer in a population-based study in Denmark, 1994–2003: summary of findings. Eur J Cancer. 2008;44:2074–2085.
- Gallus S, Lugo A, Murisic B, et al. Overweight and obesity in 16 European countries. Eur J Nutr. 2015;54:679–689.
- Zhou Y, Mendonca SC, Abel GA, et al. Variation in ‘fast-track’ referrals for suspected cancer by patient characteristic and cancer diagnosis: evidence from 670 000 patients with cancers of 35 different sites. Br J Cancer. 2018;118:24–31.
- Saarela J, Weber R. Assessment of educational misclassification in register-based data on Finnish immigrants in Sweden. Scand J Public Health. 2017;45:20–24.
- Novinson D, Puckett M, Townsend J, et al. Increasing awareness of uterine cancer risks and symptoms by using campaign materials from inside knowledge: get the facts about gynecologic cancer. J Cancer Educ. 2018. [E-pub ahead of print].
- Strömberg U, Peterson S, Holmén A, et al. Rational targeting of population groups and residential areas for colorectal cancer screening. 2018.
- de Klerk C, Gupta S, Dekker E, et al. Socioeconomic and ethnic inequities within organised colorectal cancer screening programmes worldwide. Gut. 2018;67:679–687.
- Hakama M, Malila N, Dillner J. Randomised health services studies. Int J Cancer. 2012;131:2898–2902.
- Lamin H, Eklund C, Elfstrom KM, et al. Randomised healthcare policy evaluation of organised primary human papillomavirus screening of women aged 56–60. BMJ Open. 2017;7:e014788.
- Wardle J, von Wagner C, Kralj-Hans I, et al. Effects of evidence-based strategies to reduce the socioeconomic gradient of uptake in the English NHS Bowel Cancer Screening Programme (ASCEND): four cluster-randomised controlled trials. Lancet 2016;387:751–759.
