Introduction
Genetic factors might play an important role in the development of Waldenstrom’s macroglobulinaemia (WM) and multiple myeloma (MM). Studies have shown that first-degree relatives of lymphoplasmacytic lymphoma (LPL) and WM patients have an increased risk of developing LPL/WM and other haematological malignancies [Citation1,Citation2]. Also in MM familial cases do exist [Citation3]. There are ethnic differences in the incidence of WM, MM and monoclonal gammopathy of undetermined significance (MGUS) but also geographical differences. WM is more frequent in the white population than in the Asian population and the incidence of MM is higher in African-Americans compared to the white population in the US [Citation4–6], differences that can also be the result of environmental factors. WM is associated with immune conditions such as autoimmune diseases and specific infections, but this association is not found in MM [Citation7,Citation8]. Using the Swedish Lymphoma Registry, we found a high incidence of WM in northern Sweden with aggregations of families with WM and a co-occurrence with autoimmune diseases and other haematological malignancies in 75% and 42% of the families, respectively [Citation9].
In diseases with a monoclonal immunoglobulin (paraprotein), antigenic targets of the paraproteins might also play a role in pathogenesis. Paratarg-7 (P-7), a protein of unknown function, is expressed in all human tissues and has been identified as a paraprotein target of 15% of immunoglobulin A (IgA) and immunoglobulin G (IgG) paraproteins in MGUS and MM [Citation10], and of 11% of immunoglobulin M (IgM) paraproteins in WM and MGUS of IgM type [Citation11]. In patients with an anti-P-7-specific paraprotein, the protein is hyperphosphorylated (pP-7) [Citation12]. P-7 hyperphosphorylation can be induced in wild-type P-7 (wtP-7) carriers by PKCζ and reverted by protein phosphatase 2A (PP2A). In pP-7 carriers, dephosphorylated pP-7 is defective due to inactivation of the PP2A [Citation13]. The carrier state of pP-7 is inherited in an autosomal dominant fashion and carrier of pP-7 has a higher risk for developing MM, MGUS and WM (MM: odds ratio = 7.9, p = .0001; WM: odds ratio = 6.2, p = .001) [Citation13,Citation14].
The incidence of the pP-7 carrier state is variable in patients with MGUS of IgG and Ig A type and MM: the incidence is lower in Japan and higher in African-Americans in the US compared with patients from Germany [Citation14,Citation15], but data are lacking on regional differences of pP-7 carrier state of patients with MGUS of IgM type and WM. Furthermore, the occurrence of pP-7 in familial WM is unknown. The incidence in healthy controls in Germany is 2% [Citation16].
In Sweden, the incidence of WM/LPL is high; 11.5, measured as million persons per year, with an even higher incidence in the northern counties; Norrbotten County = 17.6; and Vasterbotten County = 14.3 million persons per year [Citation17] and with an aggregation of families in these counties. This high incidence and family aggregation in one region may relate to heredity, but environmental and other unknown factors may also contribute [Citation9].
Aim
To investigate P-7 and other paratarg proteins as well as the carrier state of pP-7 in WM patients in Sweden and their relationship to the high incidence and familial clustering in the northern counties. In addition, this study investigates the inheritance pattern of the paratarg proteins.
Methods and patients
Blood and serum samples were collected after obtaining written informed consent from 42 patients with non-familial WM and from 21 patients from 12 families with two or more cases of WM, IgM MGUS and/or MM from two counties, Norrbotten and Vasterbotten. In addition, blood and serum samples were collected in seven healthy first-degree relatives from two of these families.
For two non-familial WM patients from the northern Swedish cohort, we also analysed pre-diagnostic samples from the biobank at Umeå University.
The control group included blood samples from 57 Swedish patients with WM participating in the Scandinavian Lymphoma Aetiology study (SCALE), a nationwide population-based case-control study of risk factors, including blood sampling, for malignant lymphomas carried out in Denmark and Sweden [Citation18]. The analyses of the pP-7 and the titres of the corresponding antibodies were analysed at José Carreras Centre for Immuno and Gene Therapy, Department of Internal Medicine I, Saarland University Medical School, Homburg/Saar, Germany and performed as previously described. In summary, all paratarg proteins and HSP90-SUMO were produced with an additional FLAF tag by recombinant expression in HEK293 cells and coated to Nunc maxisorb plates. Sera were diluted 1:100 and the ELISAs were performed according to standard protocols with goat anti-human Ig-mix. Paratarg-7 ELISA: Chicken anti-STOML2 (Abcam, Cambridge, UK) was coated to Nunc maxisorb plates at 4 °C overnight, before plates were blocked with 1.5% gelatine/PBS-T × 100. Whole blood cell lysate was added for 1 h at room temperature. Recombinant Fabs (10 mg/mL) specific for wild-type P-7 (wtP-7) or pP-7 were added for 1 h at room temperature, followed by anti-human-IgG-(Fab)2-biotin (1:2500) for 1 h and Strep-Pox (1:50,000 1 h RT). Between each step, intensive washing with TBS-T × 100 was performed. Development was done using o-phenylenediamine (OPD) tablets. After stopping with 3 M HCl, absorbance was measured at 490 nm on a Wallac Victor II ELISA reader (Perkin Elmer, Rodgau, Germany) [Citation12].
The serum protein electrophoreses, including immunofixation, were analysed at the local routine clinical laboratory at Sunderby Hospital.
Results
In total, 127 individuals were analysed for the carrier state of pP-7 and reactivity against P-7/pP-7 – for details see . In the cohort of non-familial WM patients from northern Sweden, 3/42 (7.1%) were found to be carriers of pP-7 and also showed reactivity against P-7/pP-7. Two of these patients had pre-diagnostic blood samples in the biobank at Umeå University. The pre-diagnostic blood sample from the first patient was collected 6.5 years before the diagnosis of WM and showed no paraprotein or reactivity against P-7/pP-7 at this time, but the patient was a carrier of pP-7. The second patient’s pre-diagnostic blood sample was collected 10 years and three months before diagnosis of WM and showed a paraprotein of IgM type (14 g/L), carrier state of pP-7 with reactivity against P-7/pP-7 in a low titre.
Table 1. Number of individuals analysed for pP-7 (n = 127).
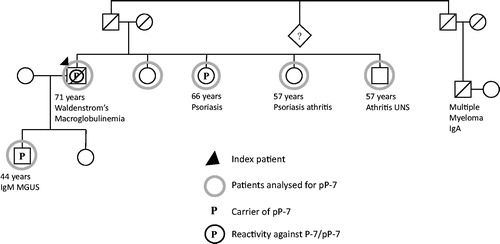
We identified 12 families in Norrbotten and Vasterbotten counties with two or more family members with WM, IgM MGUS, and/or MM and have analysed 21 affected family members for pP-7 and found that the index patients of two of the families were carriers of pP-7. In the two pP-7 positive families, five family members had a diagnosis of WM, IgM MGUS, or MM and blood samples from four of these were collected. For family 1, see . The index patient, a woman with IgM MGUS, was a carrier of pP-7 and had serum reactivity against P-7/pP-7 with a titre of 1:106. Her daughter was also a carrier of pP-7, while her son was a carrier of the protein for wild-type P-7 (wtP-7). None of the two had a paraprotein or serum antibodies against P-7/pP-7 and they had no disease. The brother of the index patient had Bence Jones Myeloma and was a carrier of pP-7, but unlike his sister, he did not have any serum antibodies against P-7/pP-7. One of his sons was a carrier of pP-7, but had no paraprotein or antibodies against P-7/pP-7, and no disease.
Figure 1. Pedigree of patient 1 with IgM MGUS with a P-7 specific IgM paraprotein carrying the pP-7.
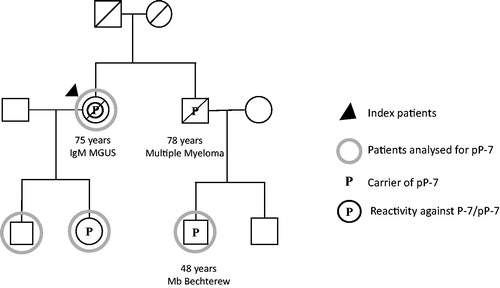
In the second family with pP-7 (), the index patient with WM was a carrier of pP-7 and he had serum reactivity against pP-7 (titre 1:106). His son had inherited the pP-7 carrier state but showed no reactivity against P-7/pP-7. He had a very small transient paraprotein of IgM type (<0.5 g/L) found at the initial family screening, but disappearing after the two-year follow-up. His daughter was carrying the protein for wtP-7, and showed no serum reactivity against P-7 or paraprotein and had no disease. This index patient had four siblings, one sister was a carrier of pP-7 while the other carried the wild-type form. None of these siblings had a paraprotein or serum antibodies against P-7/pP-7 and they had no haematological disease. The index patient had a cousin who died in a very aggressive MM, but there were no blood samples left to test.
In both families, there was a family history of autoimmune diseases. In family 1, the son to the index patient with MM had Bechterew’s disease; in family 2, two sisters had psoriasis and psoriasis arthritis, and one brother had a history of arthritis. The sister with psoriasis was a carrier of pP-7.
In the cohort of patients from the SCALE study (i.e., the control group) 5/57 (8.8%) of the patients were carriers of pP-7 and showed reactivity against P-7/pP-7. Furthermore, in the SCALE cohort, two WM patients were carriers and showed reactivity to another paratarg protein – HSP90-SUMO.
From the northern Swedish WM cohort, one patient was a carrier and showed reactivity to hyperphosphorylated paratarg-6 (pP-6) or LAPTM5 (titre 1:106). This patient had three healthy siblings and one of them carried pP-6 but without showing reactivity against pP-6. The other two siblings were carriers of wild-type of P-6 protein without reactivity or paraprotein.
Discussion
In our study, the frequency of pP-7 in all of Sweden and in the northern counties was in line with earlier studies published on patients with WM, IgM MGUS and MM from Europe. Moreover, the occurrences of pP-7 in the index patients from families with aggregation of WM, IgM MGUS and/or MM had a tendency to be higher compared with the non-familial cases (16.7% vs. 7.1%) from the same geographical area, but the results must be interpreted with caution because of small sample sizes. There is no published data about the occurrence in of pP-7 in familial WM compared to non-familial cases, only a few families with MM and MGUS are described. The inheritance of pP-7 is in most cases described in non-familial cases.
The two WM families described were mixed families with WM or IgM MGUS and MM. In the families diagnosed with only WM and IgM MGUS, the index patients did not carry pP-7. In both families, there was a family history of autoimmune diseases. A correlation between autoimmune diseases and pP-7 has not been described previously. We can show that pP-7 is inherited in these families, as described before, but further follow-up is needed to know if the family members positive for pP-7 will develop the disease.
For the first time, we were able to describe two patients who were positive for pP-7, before the diagnosis of WM (6.5 and 10 years, respectively). Before diagnosis, the first patient did not carry a paraprotein or antibodies against pP-7, but the second patient had a paraprotein of IgM type and a low titre of antibodies. Although anecdotal, these examples suggest that the chronic immune stimulation may results in a clonal evolution of B cells into WM/MGUS/MM that produces a pP-7 specific paraprotein and that the titre against pP-7 is built up over time.
In total, 11 paratarg proteins have been described [Citation19]. One of our families was a carrier of paratarg-6 (LAPTM5), and we could show that P-6 was hyperphosphorylated and inherited in the same way as described for pP-7 and other paratarg proteins.
In summary, pP-7 carrier state is a strong molecularly defined risk factor for the development of WM/MGUS and MM. The frequency of pP-7 is at the same level in non-familial WM in Sweden as in other parts of Europe, but there is a tendency that the frequency of pP-7 is higher in familial WM. The Carrier state of pP-7 can be one reason for family clustering. Positive analysis for pP-7 was shown up to 10 years before diagnosis of WM, and the hypothesis is that antibody titres against pP-7 are built up over time and with progressing disease and increasing paraprotein. The pP-7 is inherited in an autosomal dominant fashion and in families with aggregation of WM, IgM MGUS and/or MM, the finding of pP-7 enables the identification of family members at increased risk. Development of specific prophylactic strategies to inhibit the development of disease in these healthy carriers of pP‐7 require more knowledge about the mechanisms responsible for the defective dephosphorylation maintaining the hyperphosphorylated state of P-7.
Acknowledgments
The authors thank all patients and their relatives contributing to the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kristinsson SY, Bjorkholm M, Goldin LR, et al. Risk of lymphoproliferative disorders among first-degree relatives of lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia patients: a population-based study in Sweden. Blood. 2008;112:3052–3056.
- Treon SP, Hunter ZR, Aggarwal A, et al. Characterization of familial Waldenstrom's macroglobulinemia. Ann Oncol. 2006;17:488–494.
- Koura DT, Langston AA. Inherited predisposition to multiple myeloma. Ther Adv Hematol. 2013;4:291–297.
- Wang H, Chen Y, Li F, et al. Temporal and geographic variations of Waldenstrom macroglobulinemia incidence: a large population-based study. Cancer. 2012;118:3793–3800.
- Iwanaga M, Chiang CJ, Soda M, et al. Incidence of lymphoplasmacytic lymphoma/Waldenstrom's macroglobulinaemia in Japan and Taiwan population-based cancer registries, 1996-2003. Int J Cancer. 2014;134:174–180.
- Greenberg AJ, Vachon CM, Rajkumar SV. Disparities in the prevalence, pathogenesis and progression of monoclonal gammopathy of undetermined significance and multiple myeloma between blacks and whites. Leukemia. 2012;26:609–614.
- Vajdic CM, Landgren O, McMaster ML, et al. Medical history, lifestyle, family history, and occupational risk factors for lymphoplasmacytic lymphoma/Waldenstrom's macroglobulinemia: the InterLymph non-hodgkin lymphoma subtypes project. J Natl Cancer Inst Monogr. 2014;2014:87–97.
- Baecklund E, Smedby KE, Sutton LA, et al. Lymphoma development in patients with autoimmune and inflammatory disorders–what are the driving forces? Semin Cancer Biol. 2014;24:61–70.
- Brandefors L, Kimby E, Lundqvist K, et al. Familial Waldenstrom's macroglobulinemia and relation to immune defects, autoimmune diseases, and haematological malignancies – a population-based study from northern Sweden. Acta Oncol. 2016;55:91–98.
- Preuss KD, Pfreundschuh M, Ahlgrimm M, et al. A frequent target of paraproteins in the sera of patients with multiple myeloma and MGUS. Int J Cancer. 2009;125:656–661.
- Grass S, Preuss KD, Wikowicz A, et al. Hyperphosphorylated paratarg-7: a new molecularly defined risk factor for monoclonal gammopathy of undetermined significance of the IgM type and Waldenstrom macroglobulinemia. Blood. 2011;117:2918–2923.
- Grass S, Preuss KD, Pfreundschuh M. Autosomal-dominant inheritance of hyperphosphorylated paratarg-7. Lancet Oncol. 2010;11:12.
- Preuss KD, Fadle N, Regitz E, et al. Inactivation of protein-phosphatase 2A causing hyperphosphorylation of autoantigenic paraprotein targets in MGUS/MM is due to an exchange of its regulatory subunits. Int J Cancer. 2014;135:2046–2053.
- Grass S, Iida S, Wikowicz A, et al. Risk of Japanese carriers of hyperphosphorylated paratarg-7, the first autosomal-dominantly inherited risk factor for hematological neoplasms, to develop monoclonal gammopathy of undetermined significance and multiple myeloma. Cancer Sci. 2011;102:565–568.
- Zwick C, Held G, Auth M, et al. Over one-third of African-American MGUS and multiple myeloma patients are carriers of hyperphosphorylated paratarg-7, an autosomal dominantly inherited risk factor for MGUS/MM. Int J Cancer. 2014;135:934–938.
- Grass S, Preuss KD, Ahlgrimm M, et al. Association of a dominantly inherited hyperphosphorylated paraprotein target with sporadic and familial multiple myeloma and monoclonal gammopathy of undetermined significance: a case-control study. Lancet Oncol. 2009;10:950–956.
- Brandefors L, Melin B, Lindh J, et al. Prognostic factors and primary treatment for Waldenström macroglobulinemia - a Swedish Lymphoma Registry study. Br J Haematol. 2018;183:564–577.
- Smedby KE, Hjalgrim H, Melbye M, et al. Ultraviolet radiation exposure and risk of malignant lymphomas. J Natl Cancer Inst. 2005;97:199–209.
- Grass S, Preuss KD, Thome S, et al. Paraproteins of familial MGUS/multiple myeloma target family-typical antigens: hyperphosphorylation of autoantigens is a consistent finding in familial and sporadic MGUS/MM. Blood. 2011;118:635–637.