Abstract
Background: Phthalates are used as excipients in some drug products, and up to a 50-fold increased urinary excretion of phthalate metabolites compared to non-users has been demonstrated in users of such products. In vitro studies have demonstrated that phthalates stimulate mechanisms involved in gastric cancer development. We therefore examined the association between cumulative phthalate exposure from drug products and the risk of gastric adenocarcinomas.
Methods: Using the Danish Cancer Registry, we identified all patients with incident gastric adenocarcinoma from 2008 to 2015 (n = 1525). Cancer cases were matched to 10 controls. Linking information retrieved from nationwide Danish registries, we determined individual cumulative phthalate exposure to the ortho-phthalates diethyl phthalate (DEP), dibutyl phthalate (DBP) and enteric phthalate polymers from prescription drugs. The association between cumulative phthalate exposure and gastric adenocarcinoma was estimated using conditional logistic regression, adjusting for socioeconomical status and drugs or comorbidities known or suspected to modify the risk of gastric adenocarcinoma.
Results: No association was seen for the risk of gastric adenocarcinomas among individuals with high cumulative exposure to ortho-phthalates (exceeding 500 mg) (ORadj 1.22, 95% CI: 0.84–1.77). Likewise, no associations were observed individually for DEP (ORadj 1.06 95% CI: 0.63–1.76) or DBP (ORadj 1.32 95% CI: 0.78–2.23). Cumulative exposure to enteric phthalate polymers exceeding 10,000 mg, did not reveal an association with gastric adenocarcinoma (ORadj 0.79, 95% CI: 0.54–1.16) and no association was seen for individual compounds. Additionally, no dose-response pattern was observed across exposure strata (p = .39, test for trend).
Conclusion: We did not find an increased risk of gastric adenocarcinoma among Danish users of phthalate-containing drug products. Our study is limited by a low number of cases exposed to high cumulative doses of phthalates.
Introduction
Phthalates are ubiquitous compounds widely used in used in consumer care goods where they are used as plasticizers [Citation1]. Additionally, these compounds maintain color and scent in personal care goods [Citation2]. Due to the widespread use of phthalates in such products, most people are exposed. Phthalates are also used as excipients in pharmaceuticals as coating material in the production of sustained or delayed release preparations [Citation3]. They are used to prevent disintegration of the pill in the stomach acid [Citation3]. However, high exposure among users of phthalate-containing medications have been demonstrated; urine samples have revealed up to a 50-fold increased exposure among users of phthalate-containing medications compared to non-users [Citation4]. This potential harmful exposure can be prevented as all phthalate containing drug products are also represented by phthalate-free products [Citation5]. Furthermore, in vitro studies have demonstrated that some phthalates are involved in mechanisms related to carcinogenesis, such as stimulation of the aryl hydrocarbon receptor and epithelial-to-mesenchymal transition [Citation6,Citation7] mechanisms which are involved in the development of gastric adenocarcinomas [Citation8]. However, the carcinogenic properties of phthalates are still uncharacterized and human data are conflicting. Scarce data in the available literature led the U.S. Environmental Protection Agency to classify the ortho-phthalates diethyl phthalate (DEP) and dibutyl phthalate (DBP), as ‘not classifiable to human carcinogenicity’ [Citation9,Citation10].
This led us to consider if the potential extensive gastric phthalate exposure from orally administered drugs carries a risk of developing gastric adenocarcinomas. To answer this, we performed a Danish nationwide registry-based case-control study to examine the association between cumulative pharmaceutical phthalate exposure and risk of gastric adenocarcinoma.
Method
We performed a case-control study investigating the cumulative phthalate exposure from orally administered drug products among individuals diagnosed with gastric adenocarcinoma (cases) and population-based cancer-free persons (controls). The study was reported according to STROBE guidelines for observational studies [Citation11].
Data sources
Five Danish nationwide registries were used to conduct a population-based case-control study: the Danish Cancer Registry [Citation12], the National Prescription Registry [Citation13], the National Patient Register [Citation14], Registers in Statistics Denmark on educational level [Citation15] and the Civil Registration System [Citation16]. The majority of medical care in Denmark is funded by the national health authorities, this constellation allows true population-based register studies covering all Danish inhabitants. A personal identification number, a unique identifier, assigned to all Danish residents since 1968 enables linkage of individual data across registers [Citation17].
Detailed information on data sources is presented in Supplemetary Appendix A, and codes for cancer diagnoses, drug exposures and covariates are available in Supplemetary Appendix B.
The Danish Medicines Agency maintains an internal database on product-specific composition of excipients in drugs with marketing authorization from 2004 onwards. Information on type, amount and changes in composition are recorded and linked with Nordic product codes (VNR numbers) assigned to all unique drug products in the Danish formulary. Active ingredients are classified according to the Anatomic Therapeutic Chemical (ATC) index, developed by the World Health Organization [Citation18]. Linking data with the National Prescription Registry, we could quantify cumulative phthalate exposure on an individual level. Only orally administered drugs were included.
Sampling of cases and controls
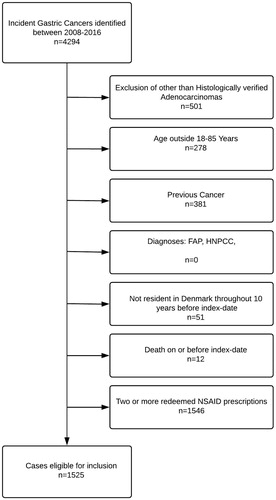
From the Danish Cancer Registry, we identified all patients (cases) in Denmark with a first-time diagnosis of gastric adenocarcinoma (ICD10: C16) in the period 2008–2015 The case population was individuals with histologically verified adenocarcinoma in the stomach. Their index date was the date of the cancer diagnosis. Exclusion criteria were age outside the range 18–85 years at the index date and any residency outside Denmark within 10 years prior to index date. Cases with a history of other cancers were excluded (except non-melanoma skin cancer) as well as cases with diagnoses associated with an increased risk of gastric cancer: hereditary nonpolyposis colon cancer [Citation19] or familial adenomatous polyposis [Citation19]. Use of non-aspirin nonsteroidal anti-inflammatory drugs (na-NSAID’s) exert chemoprotective effect against gastric cancers [Citation20] and several of the phthalate-containing drug products are na-NSAID’s [Citation5]. A study by Friis et al. [Citation21] demonstrated chemoprotective effect of na-NSAID’s among colorectal cancer patients redeeming ≥2 na-NSAID prescriptions. To avoid possible confounding from na-NSAID’s, we excluded patients with ≥2 redeemed na-NSAID prescriptions. This was, however, subjected to extensive sensitivity analysis, described below.
For each case, 10 cancer-free controls among all Danish residents of the same gender and birth year were selected, while applying the same exclusion criteria as for the cases. An index date identical to that of the corresponding case was assigned for each control. Cases were eligible for becoming controls before they became cases. Thereby, the odds ratio (OR) becomes an unbiased estimate of the incidence rate ratio that would have emerged from a cohort study based on the source population [Citation22].
Exposure definition
From the National Prescription Registry, we had information on all prescriptions redeemed by all Danish citizens from 1995 onwards. The database maintained by the Danish Medicines Agency provided detailed information on phthalate content per tablet or pill, in drug-products in Denmark from 2004 onwards. We defined exposure by the cumulative amount of ortho-phthalates or enteric phthalate polymers filled via prescription medicine during the period 2004–2015. This was done by linking information on package size and phthalate amount per tablet or pill for all dispensed prescriptions by all subjects included. Ortho-phthalate exposure was characterized by specific phthalates: DEP and DBP. Likewise, enteric phthalate polymers were characterized by specific compounds: cellulose acetate phthalate (CAP), hydroxypropylmethylcellulose phthalate (HPMCP) and polyvinyl acetate phthalate (PVAP).
Exposure was divided into strata according to the expected number of cases and controls within each stratum. Ortho-phthalate exposure was divided into three strata; <250 mg of cumulative phthalate exposure, 250–499 mg of cumulative exposure and >500 mg of cumulative exposure over the study period. For enteric phthalate polymers, the corresponding strata were <4999, 5000–9999 and >10,000 mg of cumulative exposure. Furthermore, never-exposed categories were defined for ortho-phthalates and enteric phthalate polymers respectively.
We disregarded exposure during the period – one year prior to the index date. This was done to reduce the possibility of reverse causation, while also judging that such recent exposure is unlikely to affect gastric cancer development [Citation23].
Confounding variables
The following potential confounders and risk factors were identified and incorporated in the adjusted analyses. (a) Use of drugs known or suspected to modify the risk of gastric adenocarcinoma including use of menopausal hormonal therapy, antidiabetics, drugs used to treat alcohol-related conditions, the combination metronidazole, clarithromycin and PPIs used to H. pylori eradication, NSAIDs, and Aspirin. Use of low-dose aspirin (ASA) was incorporated as a categorical variable in the adjusted analyses based on the number of redeemed prescriptions: 0, 1–15 or >15 redeemed prescriptions. (b) Comorbidities known or suspected to modify the risk of gastric adenocarcinoma: H. pylori infection, chronic atrophic gastritis, duodenal ulcers, pernicious anemia, diabetes, alcohol-related diseases, chronic obstructive pulmonary disease (COPD). (c) Socioeconomic status: educational level was incorporated as a crude measure of socioeconomic status as a categorical variable with four categories (unknown, 10 years, 11–13 year or >13 years). As in the assessment of drug exposure, we disregarded the period one year prior to the index date in the identification of confounder status (ICD-10 codes and ATC-codes are listed in Supplemetary Appendix B). This was done to reduce the possibility of reverse causation, while also judging that such recent exposure is unlikely to affect cancer development [Citation23].
Main analysis
The analysis followed a conventional matched case-control approach. We tabulated the frequency and proportion of cases and controls within categories of the exposures and covariates. We used conditional logistic regression to estimate ORs for gastric adenocarcinoma associated with high exposure to any ortho-phthalate, DEP, DBP or to any enteric phthalate polymer, adjusting for potential confounders. We performed dose–response analyses using above mentioned pre-defined exposure groups. In all analyses, exposure to any phthalate was compared with never-exposure (reference category).
Pre-planned sensitivity and sub-analyses
We examined heterogeneity of associations between phthalate exposure and gastric adenocarcinoma within strata of sex, age groups (<50, 50–69 or ≥70 years), stage of disease, history of diabetes, history of alcohol abuse or history of H. pylori infection. Further, we performed an analysis excluding lithium-treated patients because DBP exposure is mainly driven by lithium products [Citation24]. Test for trend was performed among ever-exposed to either ortho-phthalates or enteric phthalate polymers from drug products. We estimated incremental changes in OR for every 10,000 mg of ortho-phthalate or enteric phthalate polymer using logistic regression, adjusting for age, sex and confounders, risk factors and previous diagnoses listed in Supplemetary Appendix B. Further, we examined whether including or excluding all patients redeeming na-NSAID prescriptions changed our results.
Other
The study was approved by the Danish Data Protection Agency. According to Danish law, studies based solely on register data do not require approval from an ethics review board [Citation25].
Results
We included 1525 cases with gastric adenocarcinoma (). Using risk-set sampling, cases were matched by age and gender to 15,250 cancer-free population controls. The majority of included cases and controls were men (72.1%) above 50 years of age (92.1%). Ortho-phthalate exposure was similar in cases and controls; 15.3% and 14.1% had been exposed to phthalate-containing drug products. Drug use and comorbidities were balanced between cases and controls, but a larger proportion of cases (40.7%) had been diagnosed with or eradicated for H. pylori infection compared with controls (31.4%). The controls had higher educational level with 62.2% having at least 11 years of education, compared with 54.7% of the cases. The baseline characteristics of cases and controls are shown in .
Table 1. Characteristics of cases of gastric adenocarcinoma and matched population based controls.
Among the cases, 234 (15.4%) had been exposed to ortho-phthalate containing orally administered drugs during the study period compared with 2.149 (14.2%) of controls. Among these, 35 cases (2.3%) and 253 controls (1.7%) were classified as having high exposure to ortho-phthalates, which yielded an adjusted OR of 1.22 (95% CI: 0.84–1.77) for the association between high ortho-phthalate exposure and the risk of gastric adenocarcinoma ().
Table 2. Association between ortho-phthalate exposure or enteric phthalate polymer exposure and the risk of gastric adenocarcinoma within dose strata throughout the period 2004–2015.
We did not find evidence of a dose–response effect among those exposed to ortho-phthalates (p = .39, test for trend). There was no association between exposure to ortho-phthalates and the risk of gastric adenocarcinoma when specifying on DEP or DBP respectively. Likewise, there were no associations for the enteric phthalate polymers, CAP or HPMCP respectively. The few individuals exposed to PVAP did not allow for estimating the associations between exposure and risk of gastric adenocarcinoma.
Stratifying on age, sex, stage of disease as well as excluding patients diagnosed with diabetes, patients treated with lithium, patients treated for alcohol abuse, or excluding patients treated or diagnosed with H. pylori infection did not alter our findings ().
Table 3. Subgroup analysis.
We investigated whether including or excluding all patients redeeming na-NSAID prescriptions altered our results. Excluding all patients redeeming ≥1 na-NSAID prescription, left us with 712 cases and 7120 controls. Among these, 11 cases and 119 controls were exposed to >500 mg ortho-phthalate from drug products, yielding an adjusted OR of 0.81 (95% CI: 0.43–1.54). If we included all patients redeeming na-NSAID prescriptions, we had 3050 cases and 30,500 controls. Among these, 123 cases and 1052 controls were exposed to >500 mg ortho-phthalate from drug products, yielding an adjusted OR of 1.06 (95% CI: 0.86–1.30).
Discussion
In this population-based case-control study, we did not demonstrate an increased risk of gastric adenocarcinoma among individuals with high exposure to ortho-phthalates from drug products. Further, we did not find any associations between use of phthalate-containing drug products and the risk of gastric adenocarcinoma when stratifying on exposure to individual compounds or stratifying by age, gender or cancer stage. Subgroup analyses did not alter our findings and neither did including or excluding patients redeeming na-NSAID prescriptions.
The main strength of this study is the use of nationwide registries with high completeness covering all Danish inhabitants. We were able to eliminate primary non-adherence, because our data are based on dispensed prescriptions rather than issued prescriptions [Citation26]. Furthermore, a decreased impact of secondary non-adherence can be expected as exposure to phthalates was quantified in cumulative amount. The coverage of a prescription is often stretched by patients missing doses of regular medications and in this way, these patients are exposed to the entire amount of phthalates from the package [Citation27]. However, patients stopping their medications without finishing the dispensed package, are sources of misclassification.
The registries we used do not hold information on some factors known to increase the risk of gastric cancers such as smoking, alcohol consumption patterns, ethnicity, diet and body weight. However, the essentially random allocation of phthalate exposure from products with a common drug substance does not lead to the suspicion that these factors were imbalanced between our exposure groups.
Compared with other studies investigating the association between phthalate exposure and risk of cancer in humans, the use of registry data allowed large sample size and identification of up to 11 years of individual exposure history. Other epidemiological studies are based on questionnaire or biomonitoring data [Citation28–30]. Data on the carcinogenic potential of ortho-phthalates are scarce and there are no other studies on gastric cancers. Phthalates are not classified as carcinogenic substances by the U.S. environmental protection agency and the U.S. Consumer Product Safety Commission due to lack of data [Citation9,Citation10,Citation31,Citation32]. An in vitro study demonstrated that the combined presence of phthalates and 17β estradiol exerted an additive proliferative effect on MCF-7 human breast cancer cells through a downstream PI3K/Akt signaling pathway. Additionally, the combination of phthalates and 17β estradiol prevented apoptosis [Citation33]. Epidemiological studies have investigated the association between phthalate exposure and hormone-driven cancers. One study did not find an association between breast cancer in women and high urinary concentrations of monoethyl or monobutyl phthalate, the major metabolites of DEP and DBP [Citation28]. On the contrary, another study found an elevated risk of breast cancer among women with high urinary concentrations of monoethyl phthalate when compared to individuals with low concentrations [Citation29]. Occupational exposure to diethyl hexyl phthalate (DEHP) containing polyvinyl chloride has been suggested to increase the risk of testicular cancer in men [Citation30]. Several potentially harmful exposures were investigated in this study and the phthalate of interest was DEHP, which is not used in orally administered drug products. Gastric adenocarcinoma incidence is lower in women than in men, suggesting a protective effect of estrogens. Even though DEP and DBP exert weak estrogenic activity [Citation34], the mechanistic effect of ortho-phthalates in gastric adenocarcinoma development has not been investigated. However, upregulation and activation of the PI3K/Akt signaling pathway in gastric cancers have been linked to increased cell growth, proliferation, metabolism and angiogenesis in gastric cancers [Citation35].
We did not find any association between gastric adenocarcinoma and phthalate exposure from drug products. Our findings may be explained by either of two scenarios: the lack of demonstrating an association in this study could be explained by the lack of a true association between exposure to phthalates from drug products and gastric adenocarcinoma. Alternatively, if a true association exist, we might have missed it.
Gastric cancers are slowly developed [Citation36] and by using a registry with information on exposure from 2004 onwards, we might not have sufficient look-back time to capture the exposure required to demonstrate a possible effect. Even though our sample size is large compared to other studies, the finding of an OR of 1.22 (95% CI: 0.84–1.77) does not rule out a small protective or harmful effect. Judged by the upper bound of the confidence interval, the worst-case scenario would be a 77% increased risk of gastric adenocarcinoma among users of phthalate-containing drug products receiving more than 500 mg across the study period compared with population controls.
The lack of an association in this study could also be explained by factors introducing bias towards also the null. We could not account for exposure from environmental- or occupational sources in this study, but we have no reason to believe that such exposure is unequally distributed between cases and controls.
The phthalate exposure came from different drugs (List of phthalate-containing drugs used by study population in Supplementary material, ). However, confounding by indication is of minor concern, because DBP exposure mainly came from mesalazine (63.7%), lithium (26.6%) and multienzymes (9.4%) and neither inflammatory bowel disease, bipolar disease or exocrine pancreatic insufficiency, are diseases associated with lowered risk of gastric cancers [Citation37]. Most of the DEP exposure came from theophylline (82.9%), erythromycin (7.3%) and verapamil (4.1%) and neither chronic obstructive lung disease (COPD), infections/acne or atrial fibrillation, are diseases associated with lowered risk of gastric cancers [Citation37]. On the contrary, some of the diseases such as COPD, bipolar disease and exocrine pancreatic insufficiency caused by excessive use of alcohol, are diseases associated with factors known to increase the risk of gastric cancers such as smoking or low socioeconomic status [Citation38,Citation39]. Lastly, confounding introduced by drugs lowering the risk of gastric adenocarcinoma could explain the lack of an association in this study. Use of the anti-inflammatory agent mesalazine constituted the majority of DBP exposure. Chemoprotective effect of mesalazine, similar to that of NSAIDs, has not been demonstrated for gastric adenocarcinoma [Citation40] and sensitivity analyses demonstrated that use of NSAIDs did not have impact on our results.
Conclusion
We did not find an increased risk of gastric adenocarcinoma among Danish users of phthalate-containing drug products. However, our study is limited by a low number of cases exposed to high cumulative doses of phthalates.
Supplemental Material
Download MS Word (22 KB)Acknowledgments
Martin Thomsen Ernst (University of Southern Denmark) is acknowledged for help with data management.
Disclosure statement
The authors have no conflict of interest to declare. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any institutions the authors represent or funders supporting the project.
Additional information
Funding
References
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological profile for Di-n-Butyl Phthalate. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; 2002.
- Koo HJ, Lee BM. Estimated exposure to phthalates in cosmetics and risk assessment. J Toxicol Environ Health Part A. 2004;67:1901–1914.
- Aulton ME, Taylor K, editors. Aulton’s pharmaceutics: the design and manufacture of medicines. 4th ed. Edinburgh: Churchill Livingstone/Elsevier; 2013.
- Hernández-Díaz S, Mitchell AA, Kelley KE, et al. Medications as a potential source of exposure to phthalates in the U.S. population. Environ Health Perspect. 2009;117:185–189.
- Broe A, Ennis ZN, Pottegård A, et al. Population exposure to phthalate-containing drugs. Basic Clin Pharmacol Toxicol. 2017;121:153–158.
- Hsieh TH, Tsai CF, Hsu CY, et al. Phthalates stimulate the epithelial to mesenchymal transition through an HDAC6-dependent mechanism in human breast epithelial stem cells. Toxicol Sci. 2012;128:365–376.
- Hsieh TH, Tsai CF, Hsu CY, et al. Phthalates induce proliferation and invasiveness of estrogen receptor-negative breast cancer through the AhR/HDAC6/c-Myc signaling pathway. Faseb J. 2012;26:778–787.
- Peng TL, Chen J, Mao W, et al. Aryl hydrocarbon receptor pathway activation enhances gastric cancer cell invasiveness likely through a c-Jun-dependent induction of matrix metalloproteinase-9. BMC Cell Biol. 2009;10:27.
- Integrated Risk Information System (IRIS). Chemical Assessment Summary Diethyl phthalate; CASRN 84-66-2. Washington, DC: U.S. Environmental Protection Agency; 1987.
- Integrated Risk Information System (IRIS). Chemical Assessment Summary Dibutyl phthalate; CASRN 84-74-2. Washington, DC: U.S. Environmental Protection Agency; 1987.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349.
- Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39:42–45.
- Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, et al. Data Resource Profile: The Danish National Prescription Registry. Int J Epidemiol. 2017;46:798–798f.
- Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490.
- Jensen VM, Rasmussen AW. Danish Education Registers. Scand J Public Health. 2011;39:91–94.
- Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549.
- Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39:22–25.
- WHO Collaborating Centre for Drug Statistics Methodology (WHOCC). Definition and general considerations [Internet] [cited 2018 Nov 1]. Available from: https://www.whocc.no/ddd/definition_and_general_considera/
- Oliveira C, Pinheiro H, Figueiredo J, et al. Familial gastric cancer: genetic susceptibility, pathology, and implications for management. Lancet Oncol. 2015;16:e60–e70.
- Abnet CC, Freedman ND, Kamangar F, et al. Non-steroidal anti-inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta-analysis. Br J Cancer. 2009;100:551–557.
- Friis S, Riis AH, Erichsen R, et al. Low-dose aspirin or nonsteroidal anti-inflammatory drug use and colorectal cancer risk: a population-based, case-control study. Ann Intern Med. 2015;163:347–355.
- KJ. Rothman. Epidemiology An Introduction. 2nd ed. Great Clarendon Street, Oxford, UK: Oxford University Press; 2012.
- Pottegård A, Hallas J. New use of prescription drugs prior to a cancer diagnosis. Pharmacoepidemiol Drug Saf. 2017;26:223–227.
- Ennis ZN, Broe A, Pottegård A, et al. Cumulative exposure to phthalates from phthalate-containing drug products: A Danish population-wide study. Br J Clin Pharmacol 2018;84:1798–1805.
- Thygesen LC, Daasnes C, Thaulow I, et al. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39:12–16.
- Pottegård A, Christensen R, dePont Houji A, et al. Primary non-adherence in general practice: a Danish register study. Eur J Clin Pharmacol. 2014;70:757–763.
- Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58:323–337.
- Holmes AK, Koller KR, Kieszak SM, et al. Case-control study of breast cancer and exposure to synthetic environmental chemicals among Alaska Native women. Int J Circumpolar Health. 2014;73:25760.
- López-Carrillo L, Hernández-Ramírez RU, Calafat AM, et al. Exposure to phthalates and breast cancer risk in northern Mexico. Environ Health Perspect. 2010;118:539–544.
- Hardell L, Ohlson CG, Fredrikson M. Occupational exposure to polyvinyl chloride as a risk factor for testicular cancer evaluated in a case-control study. Int J Cancer. 1997;73:828–830.
- Consumer Product Safety Commission. Toxicity review for Diethyl Phthalate [Internet]. [cited 2018 Jan 19]. Available from: https://www.cpsc.gov/s3fs-public/ToxicityReviewOfDEP.pdf
- Consumer Product Safety Commission. Toxicity review for Di-n-butyl phthalate [Internet] [cited 2017 Dec 21]. Available from: https://www.cpsc.gov/s3fs-public/ToxicityReviewOfDBP.pdf
- Chen F-P, Chien M-H, Chern IY-Y. Impact of low concentrations of phthalates on the effects of 17β-estradiol in MCF-7 breast cancer cells. Taiwan J Obstet Gynecol. 2016;55:826–834.
- Harris CA, Henttu P, Parker MG, et al. The estrogenic activity of phthalate esters in vitro. Environ Health Perspect. 1997;105:802–811.
- Riquelme I, Tapia O, Espinoza JA, et al. The gene expression status of the PI3K/AKT/mTOR pathway in gastric cancer tissues and cell lines. Pathol Oncol Res. 2016;22:797–805.
- American Cancer Society. Information and Resources about for Cancer [Internet] [cited 2018 Nov 1]. Available from: https://www.cancer.org
- Chan A, Wong B. Risk factors for gastric cancer [Internet]. 2019 [cited 2019 Feb 13]. Available from: https://www.uptodate.com/contents/risk-factors-for-gastric-cancer?search=gastric%20cancer%20risk%20factors&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1
- International Agency for Research on Cancer. IARC monographs. Personal habits and indoor combustions. Tobacco smoking [Internet]. World Health Organization; 2012 [cited 2018 Nov 13]. Available from: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono100E-6.pdf
- Barker DJ, Coggon D, Osmond C, et al. Poor housing in childhood and high rates of stomach cancer in England and Wales. Br J Cancer. 1990;61:575–578.
- Ritland SR, Leighton JA, Hirsch RE, et al. Evaluation of 5-aminosalicylic acid (5-ASA) for cancer chemoprevention: lack of efficacy against nascent adenomatous polyps in the ApcMin mouse. Clin Cancer Res. 1999;5:855–863.