Introduction
Desmoid tumors (DT) are nonmalignant mesenchymal neoplasms with a high recurrence rate. Tumors concentrate into the small bowel mesentery and the abdominal wall when associated with familial adenomatous polyposis (FAP) [Citation1]. FAP patients are managed with prophylactic colon surgery to prevent the development of colorectal cancer from premalignant polyps. For these patients, DTs are an important cause of mortality accounting for approximately 10% of death causes [Citation2].
The optimal combination and sequence of different treatment strategies for DTs including surgery, radiotherapy (RT), variable medical therapies and watchful waiting remains unsolved [Citation3,Citation4]. Especially patients with progressive multiple and/or intra-abdominal DTs may benefit from systemic treatment. Medical therapy includes hormonal therapy, nonsteroidal anti-inflammatory drugs, tyrosine kinase inhibitors, and chemotherapy.
The pathophysiology of DTs involve characteristic β-catenin accumulation in tumor cells. The abundant β-catenin in the nucleus acts as a co-regulator for transcription factors controlling target genes such as cyclin D1 CCND1. Cyclin D1 in complex with cyclin-dependent kinases (cdk) 4 and 6 convey cell-cycle progression from G1 to S phase. Uncontrolled proliferation leads to tumorigenesis. Palbociclib, ribociclib, and abemaciclib are European Medicines Agency and U.S. Food and Drug Administration approved cdk 4/6 inhibitors for advanced breast cancer used in combination with hormonal therapy [Citation5]. In phase-II study of palbociclib in well differentiated or dedifferentiated liposarcoma, the median progression free survival (PFS) was 17.9 weeks with one complete response noted [Citation6].
The effect of systemic treatments in DTs is inconsistent, calling for novel approaches. We report here a prolonged disease stabilization with ribociclib combined with goserelin and letrozole in a patient with multiple aggressive DTs resistant to endocrine treatment alone.
Case report
A 19-year-old otherwise healthy female was diagnosed with multifocal DTs. She had a familial history of FAP and a colectomy was performed previously. During the long treatment of this patient, management guidelines of DTs have changed toward a more conservative approach. In this patient, six DTs were operated in eight operations, one recurrent tumor was resected six times. Due to rapid growth, three DTs were treated with four separate RT courses and one tumor was irradiated twice. Fractionation schemes were 50 Gy, 60 Gy, 40 Gy and 60 Gy, all in 2 Gy fractions. The patient was treated with tamoxifen, oral vinorelbine, pegylated liposomal doxorubicin, toremifen, toremifene combined with goserelin, and letrozole combined with goserelin. The DTs did not respond to cytotoxic drugs nor tamoxifen. Partial response (toremifene) or prolonged stable disease was achieved by the other endocrine treatments (Supplementary Table 1).
Upon progression during goserelin and letrozole therapy in January 2017 ribociclib 600 mg was added. During this treatment, pain was relieved and the total dose of opioids was diminished. The best response according to World Health Organization (WHO) criteria was stable disease with maximal size reduction of 18% in the sum of the product of bi-dimensional measurements (). WHO criteria were used instead of RECIST 1.1. criteria due to the irregular tumor shape of most lesions. Ribociclib caused grade 1 fatique, a tickling sensation in the tumors, grade 1 oral mucositis, acne-like rash in the neck, and vesicular hand rash. Due to grade 4 neutropenia, the dose was reduced to 400 mg and then further to 200 mg. In October, grade 4 neutropenia recurred, the pain was increased and one lesion enlarged significantly, and ribociclib was discontinued though formal criteria for progressive disease were not reached. In November, a growing pleural tumor was irradiated with 30 Gy in 3 Gy fractions.
Figure 1. Ribociclib dose and desmoid tumor perpendicular diameters according to WHO criteria before and after the initiation of medication.
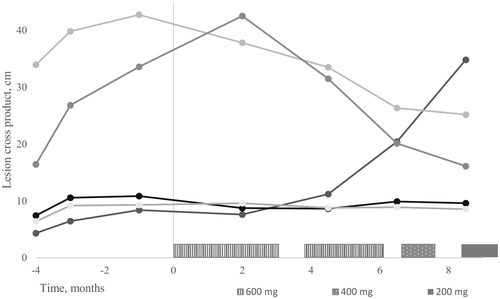
In December 2017, blood tests revealed deep leucopenia 0.8 109/l and trombocytopenia 64 g 109/l. A bone marrow aspirate revealed promyelocytic leukemia. Fluorescent in situ hybridization confirmed the diagnostic PML-RARA fusion gene. At the time of writing this report (October 2018), maintenance leukemia treatment was finished with morphological and molecular remission in the bone marrow. The DTs continued to grow slowly except for earlier irradiated tumors which were shrinking. Fulvestrant was initiated.
A radiologist (A.B.) with oncologist (K.S.) assessed the responses according to WHO and RECIST 1.1 criteria [Citation7]. Non-irradiated DTs and irradiated tumors progressing after radiotherapy were considered evaluable. The patient gave informed consent for the experimental therapy with ribociclib and publication of this report.
Discussion
We report here a patient with multiple DTs not amenable to surgery treated with ribociclib combined with goserelin and letrozole. The therapy continued for 9 months with stable disease, decrease in size of several tumor lesions and symptomatic relief. To our knowledge, this is the first report of ribociclib therapy for DTs.
Several facts indicate that sex hormones influence the growth of DTs. The incidence of DTs is higher in women at childbearing age. Pregnancy and sex steroids may promote the development of DTs [Citation8]. Estrogen receptor (ER) β is positive in up to 100% of DTs; however, the expression has not been associated with treatment response [Citation9,Citation10]. Tamoxifen is the most commonly used selective estrogen-receptor modulator and toremifene has also shown activity as first- and second-line endocrine therapy of DTs [Citation11,Citation12]. A systematic review of hormonal therapy reported a response rate (RR) of 51% at a median treatment duration of 9 months. The review was based on case reports and small series and therefore publication bias cannot be excluded [Citation13]. In a prospective phase-II study of 59 patients, the Children’s Oncology Group only 8% responded and 36% were progression free at 2 years [Citation14]. Goserelin and aromatase inhibitors have reported to have activity in DTs in a few case reports [Citation15–17]. Furthermore, endocrine treatments have a favorable toxicity profile.
In ER-positive breast cancer cyclin D1 protein overexpression has been associated with poor prognosis [Citation18,Citation19]. This may be mediated by the connection of cyclin D1 expression and hormone therapy resistance [Citation20]. Ribociclib is a highly specific cdk 4/6 inhibitor that binds to the complex of cyclin D1 and cdk 4 or 6 and thereby induces cell cycle arrest. Cdk 4/6 inhibitors have exhibited high efficacy in advanced ER positive breast cancer by substantial PFS prolongation [Citation5,Citation21]. In DTs, β-catenin accumulation has been associated with cyclin D1 overexpression [Citation22]. Cyclin D1 expression has varied in different series from 44% to 71% [Citation22–24]. In breast cancer, cdk 4/6 inhibitors act synergistically with antiestrogen treatment [Citation5]. Moreover, several lines of evidence indicate estrogen dependence of DTs. These considerations motivated the experimental use of ribociclib combined with endocrine treatment in this treatment resistant DT patient.
Ribociclib combined with goserelin and letrozole led to symptomatic relief and stabilization of multiple DTs. Although the patient experienced a brief objective response previously on toremifene, none of the previous six systemic treatments stabilized the disease for as long as 10 months. The disease had previously progressed during treatment with goserelin and letrozole, indicating that addition of ribociclib was responsive for pain relief and a prolonged disease stabilization. Although spontaneous regression cannot be excluded, this remains unlikely as none of the patients multiple DTs had previously spontaneously regressed. Moreover, only lesions progressing at start of treatment were included in the response evaluation. Bi-dimensional measurements of five evaluable lesions indicated mixed response, with the largest decreasing in size and others remaining stable. Different clonal origin of the tumors may be responsible for the heterogenous treatment response. Measurement of the DT size even from high-quality MRI images is often difficult due to the irregular shapes of the tumors and their poorly circumscribed border. For this reason, we chose to use WHO response criteria instead of the more commonly used RECIST criteria. Ribociclib dose had to be reduced repeatedly and the treatment was eventually discontinued due to cytopenia and fatigue, which were interpreted as side effects of the drug. The most common adverse events of cdk 4/6 inhibitors are hematologic and require interruption and dose modifications [Citation25]. In this patient, leukopenia and thrombocytopenia increased soon after the treatment discontinuation and the patient was diagnosed with acute promyelocytic leukemia (APL). In retrospect, APL may have contributed to the increasing myelosuppression during ribociclib treatment even earlier. APL occurring after cancer treatments is a rare event representing approximately 11% of new APL patients [Citation26]. We have found no evidence indicating that ALP might be complication of ribociclib or any other cdk 4/6 inhibitor [Citation25,Citation27]. Therapy-related APL may be associated with previous radiation or cytotoxic therapy, particularly topoisomerase II inhibitors. The patient had received three courses of liposomal doxorubicin and received several radiotherapies. Of these radiotherapy is possibly the primary contributing factor to the development of leukemia. Therapy-related APL is managed like de novo leukemia and the prognosis is fairly good [Citation26]. Especially when treating young DT patients with cytotoxic agents or radiotherapy the risk for a secondary cancer should be taken into account.
Conclusion
In conclusion, ribociclib in combination with endocrine treatment may have activity in DTs. Our experience promts for further investigation of ribociclib in DTs.
Disclosure of interest
Ribociclib was provided by Novartis.
Supplemental Material
Download MS Word (16.6 KB)Acknowledgments
We want to thank Novartis for providing ribociclib for this study.
Additional information
Funding
References
- Heiskanen I, Jarvinen HJ. Occurrence of desmoid tumours in familial adenomatous polyposis and results of treatment. Int J Colorectal Dis. 1996;11:157–162.
- Koskenvuo L, Pitkaniemi J, Rantanen M, et al. Impact of screening on survival in familial adenomatous polyposis. J Clin Gastroenterol. 2016;50:40–44.
- Yao X, Corbett T, Gupta AA, et al. A systematic review of active treatment options in patients with desmoid tumours. Curr Oncol. 2014;21:613–629.
- ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2014;25:iii102–iii112.
- Abraham J, Coleman R, Elias A, et al. Use of cyclin-dependent kinase (CDK) 4/6 inhibitors for hormone receptor-positive, human epidermal growth factor receptor 2-negative, metastatic breast cancer: a roundtable discussion by The Breast Cancer Therapy Expert Group (BCTEG). Breast Cancer Res Treat. 2018;171:11–20.
- Dickson MA, Tap WD, Keohan ML, et al. Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. JCO. 2013;131:2024–2028.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247.
- Fiore M, Coppola S, Cannell AJ, et al. Desmoid-type fibromatosis and pregnancy: a multi-institutional analysis of recurrence and obstetric risk. Ann Surg. 2014;259:973–978.
- Santos GA, Cunha IW, Rocha RM, et al. Evaluation of estrogen receptor alpha, estrogen receptor beta, progesterone receptor, and cKIT expression in desmoids tumors and their role in determining treatment options. Biosci Trends. 2010;4:25–30.
- Mignemi NA, Itani DM, Fasig JH, et al. Signal transduction pathway analysis in desmoid-type fibromatosis: transforming growth factor-beta, COX2 and sex steroid receptors. Cancer Sci. 2012;103:2173–2180.
- Brooks MD, Ebbs SR, Colletta AA, et al. Desmoid tumours treated with triphenylethylenes. Eur J Cancer. 1992;28A:10148.
- Fiore M, Colombo C, Radaelli S, et al. Hormonal manipulation with toremifene in sporadic desmoid-type fibromatosis. Eur J Cancer. 2015;51:2800–2807.
- Bocale D, Rotelli MT, Cavallini A, et al. Anti-oestrogen therapy in the treatment of desmoid tumours: a systematic review. Colorectal Dis. 2011;13:e388–e395.
- Skapek SX, Anderson JR, Hill DA, et al. Safety and efficacy of high-dose tamoxifen and sulindac for desmoid tumor in children: results of a Children’s Oncology Group (COG) phase II study. Pediatr Blood Cancer. 2013;60:1108–1112.
- Bauernhofer T, Stoger H, Schmid M, et al. Sequential treatment of recurrent mesenteric desmoid tumor. Cancer 1996;77:1061–1065.
- Wilcken N, Tattersall MH. Endocrine therapy for desmoid tumors. Cancer. 1991;68:1384–1388.
- Debled M, Le Loarer F, Callonnec F, et al. Complete response to exemestane in a patient with a desmoid tumor. Future Oncol. 2012;8:483–486.
- Ahlin C, Lundgren C, Embretsen-Varro E, et al. High expression of cyclin D1 is associated to high proliferation rate and increased risk of mortality in women with ER-positive but not in ER-negative breast cancers. Breast Cancer Res Treat. 2017;164:667–678.
- Xu XL, Chen SZ, Chen W, et al. The impact of cyclin D1 overexpression on the prognosis of ER-positive breast cancers: a meta-analysis. Breast Cancer Res Treat. 2013;139:329–339.
- Thangavel C, Dean JL, Ertel A, et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer. 2011;18:333–345.
- Spazzapan S, Conte P, Simoncini E, et al. C11Updated results from MONALEESA-2, a phase 3 trial of first-line ribociclib. + letrozole in hormone receptor-positive (HR+), HER2-negative (HER2–) advanced breast cancer (ABC). Ann Oncol. 2017;28:mdx424.010–mdx424.010.
- Saito T, Oda Y, Tanaka K, et al. beta-catenin nuclear expression correlates with cyclin D1 overexpression in sporadic desmoid tumours. J Pathol. 2001;195:222–228.
- Jilong Y, Jian W, Xiaoyan Z, et al. Analysis of APC/beta-catenin genes mutations and Wnt signalling pathway in desmoid-type fibromatosis. Pathology 2007;39:319–325.
- Colombo C, Foo WC, Whiting D, et al. FAP-related desmoid tumors: a series of 44 patients evaluated in a cancer referral center. Histol Histopathol. 2012;27:641–649.
- Spring LM, Zangardi ML, Moy B, et al. Clinical management of potential toxicities and drug interactions related to cyclin-dependent kinase 4/6 inhibitors in breast cancer: practical considerations and recommendations. Oncologist. 2017;22:1039–1048.
- Yin CC, Glassman AB, Lin P, et al. Morphologic, cytogenetic, and molecular abnormalities in therapy-related acute promyelocytic leukemia. Am J Clin Pathol. 2005;123:840–848.
- Kassem L, Shohdy KS, Lasheen S, et al. Hematological adverse effects in breast cancer patients treated with cyclin-dependent kinase 4 and 6 inhibitors: a systematic review and meta-analysis. Breast Cancer. 2018;25:17–27.
