Erdheim–Chester Disease (ECD) is a rare, non-Langerhans histiocytosis, characterized by the accumulation of foamy macrophages, chronic inflammation, fibrosis and organ failure [Citation1,Citation2]. Interferon alfa (IFNα) is the most commonly prescribed empiric treatment for ECD patients. However, efficacy varies among patients and according to the sites of disease involvement [Citation3,Citation4]. In addition, prolonged treatment with IFNα is often poorly tolerated [Citation3,Citation4].
The BRAFV600E mutation is found in more than 50% of ECD cases [Citation5,Citation6]. Targeted therapy with the BRAF-inhibitor vemurafenib showed impressive, reproducible and sustained efficacy in patients with BRAFV600E-mutated ECD disease [Citation7,Citation8]. However, the frequent occurrence of serious, sometimes life-threatening, adverse events with vemurafenib are of significant concern [Citation7–9]. Because of these limitations, there is an ongoing search for potentially efficacious but less toxic therapies.
Aberrant mechanistic Target of Rapamycin (mTOR) activation is present in neoplastic and inflammatory conditions [Citation10–13]. Because of its antiproliferative and immunosuppressive properties, mTOR inhibitors are used in several conditions such as malignancies, rheumatic diseases, and in the prevention of allograft rejection [Citation10–13]. Gianfreda et al. recently reported promising results with sirolimus (SRL), a prototype drug for all mTOR inhibitors, combined with prednisone (PDN) in ECD patients [Citation14].
However, because SRL was combined with corticosteroids (CSs), it is difficult to ascertain the efficacy of SRL itself on the disease process. In an accompanying editorial, the need for more data on SRL, particularly when given as monotherapy, was emphasized [Citation15]. To date, however, we could not find such data in the literature.
Herein, we describe our results with SRL monotherapy in 3 consecutive ECD patients. Two patients were treatment-naive, one patient had received prior treatment with PDN.
Case presentations
Case 1
A 75-year-old man was referred to our center for further analysis of suspected progressive multisystem disease. Three months earlier he had been hospitalized elsewhere for profound pericardial effusion, for which pericardiocentesis was performed. IgG4-related disease (IgG4-RD) was suspected and the patient was started on initial high-dose PDN, subsequently tapered to a maintenance dose of 10 mg/day. At the time of referral, he complained of severe long-standing fatigue, leg weakness and dyspnea. Dyspnea had regressed somewhat after the drainage procedure but otherwise, he had felt no clinical improvement following initiation of PDN treatment. His medical history revealed diabetes type 2, chronic renal failure, chronic atrial fibrillation and coronary sclerosis. Six years prior to referral he was analyzed elsewhere for a bilateral perirenal mass, complicated by a left-sided afunctional hydronephrotic kidney, for which nephrectomy was performed. Histopathological examination had revealed extensive chronic xanthogranulomatous inflammation. No specific medical treatment was started. Laboratory investigation showed signs of chronic inflammation and hypogonadism (). Thoraco-abdominal CT scan showed profound residual pericardial effusion, ground-glass opacities, thickened interlobular septa and patchy consolidations in both lungs, and right-sided ‘hairy kidney’. Total body 18F-labeled fluorodeoxyglucose positron emission tomography (18FDG- PET) scanning showed intense uptake in the right kidney but was otherwise normal. 99Tc-bone scintigraphy showed bilateral increased symmetrical osteoblastic activity in proximal tibia and distal femur. Brain MRI showed hyperintense lesions in cerebellar peduncles, expanding into both cerebellar hemispheres, and in the dentate nuclei. Histopathological reevaluation of specimens from earlier nephrectomy showed abundant fibrous tissue with a population of infiltrating foamy histiocytes (). BRAFV600E mutation in biopsy specimen tested positive (). Combined findings were compatible with a diagnosis of ECD. Treatment was started with SRL 3 mg/day orally (single daily dose), subsequently increased to 4 mg/day to achieve target blood trough levels between 8 and 12 ng/mL [Citation14]. Maintenance dose of PDN 10 mg/day was tapered and subsequently discontinued. After 4 months of SRL treatment, the patient noted a definite improvement in subjective well-being. Dyspnea had disappeared. He also noted an increase in walking ability. WBC had normalized (8.7 × 109/L). Repeat radiological and nuclear examination at 6 months follow-up (FU) showed clear regression of pericardial effusion (), but otherwise stable disease. SRL treatment was well tolerated. Repeat radiological and nuclear examination at one year FU, with the patient reporting clinically persistent improvement in subjective well-being and walking ability, showed further regression and less density of remaining pericardial effusion () and some decrease in osteoblastic activity in tibia and femur. However, an increase in patchy consolidations in both lungs and right-sided pleural effusion was noted. Two months later, at 14 months FU, the patient developed thoracic pain and a persistent cough. This was followed by rapid clinical deterioration due to progressive pulmonary ECD disease, from which he eventually succumbed. Autopsy was not performed.
Figure 1. Typical histopathologic findings of ECD and detection of BRAFV600E mutation (case 1). Tissue sample of the perinephric mass is characterized by an extensive xanthogranulomatous fibro-inflammatory reaction. Numerous foamy macrophages are present, accompanied by fibrosis and lymphoplasmacytic cell infiltration (A). Immunohistologically, macrophages were strongly positive for CD68 (B) and Factor XIIIa (C) and negative for CD1a (D) and S100 (E). The BRAFV600E mutation was detected with a BRAFV600E mutation-specific antibody (Clone VE1, Spring Bioscience, Pleasanton, CA) (F). The mutation was confirmed by PCR amplification at amino acid position 600 with an in house protocol and subsequent sequencing using the Pyromark Q24 (QIAGEN, Hilden, Germany, detection limit 10%). Response to treatment assessed by repeated cardiac MRI scanning in case 1. Cardiac MRI scan at presentation (G) and after 6 months (H) and 12 months (I) of SRL treatment, respectively, showed clear regression of pericardial effusion.
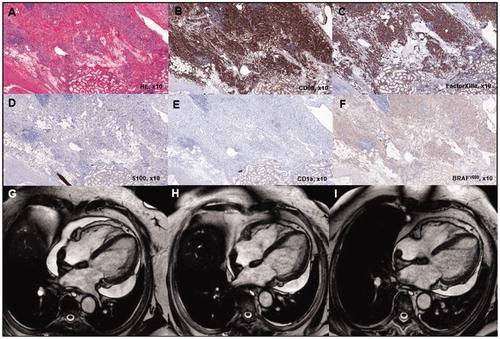
Table 1. Relevant laboratory data of ECD patients on admission.
Case 2
A 70-year-old male was referred to our center for further analyses of radiological atypical but presumed biopsy-proven retroperitoneal fibrosis. He had complaints of fatigue and chronic abdominal pain for several years. He had a history of hypertension, diabetes type 2 and ocular-ischemic syndrome. Physical examination was unremarkable. Laboratory investigation showed signs of chronic inflammation (). Abdominal CT scanning showed a bilateral perirenal mass with no concurrent periaortic or parailiacal mass (). Tissue specimens from CT-guided biopsy of the right-sided perirenal mass performed elsewhere were reexamined. A diffuse infiltrate with CD68 positive, Factor 13a positive, S100 negative and CD1a negative cells were found. Insufficient tissue material was available to perform BRAFV600E mutation analysis, however, BRAFV600E mutation analysis in plasma proved positive. Results were compatible with a diagnosis of ECD. Additional whole body 18FDG PET-scan showed avidity of the bilateral perirenal mass but otherwise no FDG-avid lesions elsewhere. Skeletal scintigraphy did not show increased osteoblastic activity at tibia or femora. MRI brain and cardiac MRI showed no signs of CNS or cardiac involvement of ECD. Treatment with SRL 2 mg/day was started. Because of the difficulty to control diabetes and severe atherosclerotic disease, no CSs were added to this treatment regimen. After 3 months of SRL treatment, abdominal pain had gradually subsided almost completely. WBC (11.7 × 109/L) and platelet count (377 × 109/L) were (near) normalized. After 6 months of SRL therapy, a repeat CT scan showed marked regression of the bilateral perirenal mass (). Two months later, the patient discontinued SRL without consulting his treating physician, presuming that his newly developed knee pain was a side effect of SRL. Six months later, at 14 months FU, abdominal pain had returned. Repeat CT scanning showed an increase in size and density of the bilateral perirenal masses compared to the CT scan at 6 months FU (). Treatment with SRL was restarted (2 mg/day). Again, abdominal pain subsided gradually and a repeat CT scan 6 months later showed a clear response with a decline in size and density of the bilateral perirenal mass (). Further detailed FU was complicated by significant cognitive deterioration, presumably due to developing vascular dementia.
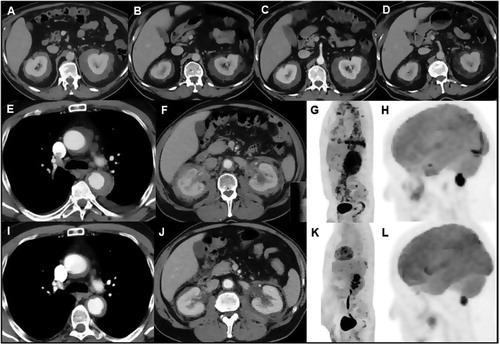
Figure 2. Response to treatment assessed by repeated contrast-enhanced CT scanning in case 2. Abdominal CT scan at presentation (A) and after 6 months of sirolimus (SRL) treatment showed a marked decrease in size and density of the bilateral perirenal soft tissue mass (B). Repeat CT scan 6 months after self-discontinuation of SRL treatment, 8 months after the first follow-up CT scan, showed an increase in size and density of the bilateral perirenal mass (C). Repeat CT scan 6 months after reinstituting SRL treatment again showed a marked decrease in size and density of the bilateral perirenal mass (D). Response to treatment assessed by different imaging modalities in case 3. Thoraco-abdominal CT scan performed at presentation (E,F) and after 6 months of SRL treatment showed a marked decrease in size of the periaortic (I) and perirenal (J) soft tissue mass. 18FDG-PET scan at presentation (G,H) and after 6 months of SRL treatment showed disappearance of 18FDG-uptake at the level of the thoracic (aortic arch) and abdominal aorta, pleuropericardium and bilateral perirenal mass (K) and marked decrease of/disappearance in 18FDG-uptake at the level of the pons and extra-axial lesion near the foramen magna (L), respectively.
Case 3
A 61-year-old man was referred to our tertiary care center for analysis of suspected bilateral perirenal mass on ultrasonography. He complained of general malaise, loss of appetite, loin pain and weight loss of 4 kg in 2 months. He had a history of urolithiasis, depression, hypertension and TIA of the right hemisphere one year before presentation. Physical examination was unremarkable, except for hypertension (BP: 164/92 mm Hg). Laboratory investigation showed clear signs of (chronic) inflammation and hypogonadism (). Thoraco-abdominal CT scan showed periaortic soft tissue encasement extending from the distal aorta ascendens () to the proximal iliacal arteries, bilateral pleural effusion, pericardial effusion and a bilateral perirenal soft tissue mass without hydronephrosis (). Patchy sclerotic skeletal abnormalities were present on skeletal scintigraphy. 18FDG- PET scanning showed increased uptake in aforementioned perivascular abnormalities, pleuropericardium (), cerebral pons and extra-axial near the foramen magna (), distal femur and proximal tibia on both sides. MRI of the heart was suggestive for aortic arc fibrosis, there was no cardiac involvement. Brain MRI confirmed an extra-axial lesion near the foramen magna with mild compression on the brainstem/cervical myelum. Histopathological examination of CT-guided needle biopsy of the perirenal mass showed fibrosis, patchy infiltrate consisting of lymphocytes, histiocytes and plasma cells. Histiocytic cells were strongly positive for CD68 and Factor XIIIa, and negative for CD1a and S100. BRAFV600E mutation in biopsy specimen tested negative. A diagnosis of BRAF-negative ECD was made and treatment was started with SRL 3 mg/day, subsequently increased to 4 mg/day to achieve target blood levels between 8 and 12 ng/ml. Because of his medical history (depression, difficult to control hypertension, TIA) no CSs were added. SRL treatment resulted in a remarkable clinical and radiological improvement (). SRL treatment was well tolerated. Laboratory abnormalities improved, repeated CT and total body PET scanning at 6 months and 12 months FU showed persistent objective responses at all sites, except for the bone abnormalities (). At 6 months FU, asymptomatic noninfectious interstitial pneumonitis (NIP) was observed, which resolved after dose reduction of SRL to 3 mg/day. At the time of his latest FU, 25 months after presentation, he feels well with no reported clinical side effects of SRL treatment (3 mg/day).
Discussion
ECD is an extremely rare and multifaceted disorder and may have a progressive, life-threatening course [Citation1,Citation2]. There is an ongoing search for potentially efficacious but less toxic therapies than long-term IFNα for wild-type ECD and for BRAF-mutated ECD with significant co-morbidities in whom vemurafenib may not be an option. These therapies are typically targeted at cytokines or their receptors and include infliximab, imatinib, anakinra and cladribine [Citation16,Citation17].
Another potentially viable targeted therapy in patients with ECD may be SRL. Based on the assumption that ECD has an inflammatory-neoplastic nature, Gianfreda et al. treated 10 ECD patients with combined SRL and PDN [Citation14]. Study patients initially received PDN at 0.75 mg/kg per day, tapered to 5–2.5 mg/day by month 6. Target SRL blood levels were 8–12 ng/mL. Treatment was continued for at least 24 months in patients who showed disease stabilization or improvement. Eight patients achieved stable disease or objective responses, whereas 2 had disease progression. Two patients died of progressive CNS disease and small-cell lung cancer, respectively. Overall, treatment was well tolerated.
We observed an objective positive response to oral SRL monotherapy in all 3 ECD patients, using the SRL target levels and FU protocol of the study of Gianfreda et al. [Citation14] Clinical improvement was followed by radiological-documented improvement, particularly in case 2 and 3 in whom significant regression of perirenal (case 2, 3) and periaortic involvement (case 3) was observed. Regression or disappearance of pericardial (case 1, 3) and pleural effusion (case 3) was also observed in those with such involvement. In case 3, CNS involvement also regressed. No clear improvement of bone activity was noted in our study patients. In the study of Gianfreda et al., positive treatment responses were also particularly observed at the retroperitoneum (5/8) and cardiovascular (3/4) and to lesser extent bone (3/9) and CNS (1/3) [Citation14]. Efficacy of SRL was further confirmed by the ‘rechallenge’ with SRL in one of our patients (case 2), which again led to amelioration of pain and CT-documented regression of the bilateral perirenal mass.
Overall, treatment was well tolerated with no significant clinical side effects and patients were able to continue it chronically for several years. Studies with SRL in other patients, particularly those after kidney transplantation, have shown its tolerability and safety [Citation18–20]. Most side-effects seem to be dose-dependent and include hyperlipidemia, diabetes mellitus, edema, stomatitis and oral ulcerations, and NIP [Citation18–20]. The latter complication shows a varying incidence of 5% up to 39% [Citation18,Citation19]. These side effects typically resolve after dose reduction or temporary withdrawal [Citation18,Citation19]. After 6 months SRL therapy, asymptomatic NIP was observed in 1 of our 3 patients (case 3), which resolved after dose reduction. In the study of Gianfreda et al., 1 of 10 patients treated with SRL combined with PDN developed asymptomatic NIP, which resolved after temporary withdrawal and subsequent resuming of SRL at a lower dose [Citation14].
One of our patients (case 1) eventually died of progressive pulmonary ECD disease. It should be noted that we established the diagnosis of multisystem ECD in this patient on revision of biopsy material from 6 years earlier. At that time, nephrectomy was performed but otherwise, no specific medical treatment was instituted. Hence, this patient presented to us with long-standing untreated multisystem ECD except for a recent unsuccessful course of CS treatment.
SRL mechanism of action is to bind to FKB12 to inhibit a subunit of mTOR, mTORC1 [Citation12,Citation21]. Prolonged exposure of SRL may reduce the activity of mTORC2 [Citation12,Citation21]. mTOR is a serine/threonine kinase that regulates cell growth, proliferation and survival. mTOR is identified as a component of two interacting complexes, mTORC1 and mTORC2, that regulate T-cell lineage specification and macrophage differentiation. mTORC1 drives the proinflammatory expansion of specific T helper cells, both mTORC1 and mTORC2 inhibit the development of specific regulatory T cells. Indirectly, mTORC2 favors expansion of specific T helper cells promoting B-cell activation and auto-antibody production. Outside the immune system, mTORC1 also controls fibroblast proliferation with implications for tissue fibrosis [Citation12,Citation21–23].
In biopsy specimen of ECD patients, infiltrating histiocytes showed strong expression of the phosphorylated forms of mTOR and of its downstream kinase p70S6K, indicating mTOR pathway activity with particular involvement of mTORC1 [Citation14]. In another series of ECD patients, PIK3CA mutations leading to mTOR activation were found in 11% of patients [Citation24]. These findings underline the role of aberrant mTOR activation in ECD pathogenesis and its potential for targeted therapy with SRL in this disorder.
As CSs may not be effective in ECD, except for possibly regimens using high doses, the need for data on mTOR inhibitors given as monotherapy was emphasized [Citation14,Citation15]. The present data illustrate the efficacy of the mTOR inhibitor by itself, thereby underlining the potential for prescribing SRL as monotherapy in selected ECD patients.
Conclusion
We observed a positive response to SRL monotherapy in 3 consecutive ECD patients with subjective clinical improvement and radiological-documented regression of organ involvement. Treatment was well tolerated. SRL monotherapy may be an option in ECD patients with contra-indications for or intolerance to first-line IFNα therapy or maybe as an initial step in mild ECD disease, regardless of mutational status. However, additional cases and further follow-up studies are needed to assess more clearly its potential as monotherapy.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Haroche J, Arnaud L, Cohen-Aubart F, et al. Erdheim-Chester disease. Curr Rheumatol Rep. 2014;16:412.
- Estrada-Veras JI, O’Brien KJ, Boyd LC, et al. The clinical spectrum of Erdheim-Chester disease: an observational cohort study. Blood Adv. 2017;1:357–366.
- Hervier B, Arnaud L, Charlotte F, et al. Treatment of Erdheim-Chester disease with long-term high-dose interferon-α. Semin Arthritis Rheum. 2012;41:907–913.
- Arnaud L, Hervier B, N´eel A, et al. CNS involvement and treatment with interferon-α are independent prognostic factors in Erdheim Chester disease: a multicenter survival analysis of 53 patients. Blood. 2011;117:2778–2782.
- Haroche J, Amoura Z, Trad SG, et al. Vaiability I the efficacy of interferon-α in Erdheim-Chester disease by patient and site of involvement. Arthritis Rheum. 2006;54:3330–3336.
- Haroche J, Charlotte F, Arnaud L, et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120:2700–2703.
- Haroche J, Cohen-Aubart F, Emile JF, et al. Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAF(V600E)-mutated Erdheim-Chester disease. Jco. 2015;33:411–418.
- Diamond EL, Subbiah V, Lockhart AC, et al. Vemurafenib for BRAF V600-mutant Erdheim-Chester disease and langerhans cell histiocytosis: analysis of data from the histology-independent, phase 2, open-label VE-BASKET study. JAMA Oncol. 2018;4:384–388.
- Vaglio A, Diamond EL. Erdheim-Chester disease: the "targeted" revolution (editorial). Blood. 2017;130:1282–1284.
- Powell JD, Pollizzi KN, Heikamp EB, et al. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68.
- Yuan R, Kay A, Berg WJ, et al. Targeting tumorigenesis: development and use of mTOR inhibitors in cancer therapy. J Hematol Oncol. 2009;2:45.
- Perl A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat Rev Rheumatol. 2016;12:169–182.
- McCormack FX, Inoue Y, Moss J, National Institutes of Health Rare Lung DiseasesConsortium; MILES Trial Group, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606.
- Gianfreda D, Nicastro M, Galetti M, et al. Sirolimus plus prednisone for Erdheim-Chester disease: an open-label trial. Blood. 2015;126:1163–1171.
- Haroche J, Amoura Z. mTOR: a new target in Erdheim-Chester disease?. Blood. 2015;126:1151–1152.
- Goyal G, Shah MV, Call TG, et al. Efficacy of biological agents in the treatment of Erdheim-Chester disease. Br J Haematol. 2018;183:520–524.
- Graziani G, Podestà MA, Cucchiari D, et al. Erdheim-Chester disease: from palliative care to targeted treatment. Clin Kidney J. 2014;7:339–343.
- Ventura-Aguiar P, Campistol JM, Diekmann F. Safety of mTOR inhibitors in adult solid organ transplantation. Exp Opin Drug Saf. 2016;15:303–319.
- Sadowski K, Kotulska K, Jóźwiak S. Management of side effects of mTOR inhibitors in tuberous sclerosis patients. Pharmacol Rep. 2016;68:536–542.
- Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando). 2014;28:126–133.
- Hillel AT, Gelbard A. Unleashing rapamycin in fibrosis (editorial). Oncotarget. 2015;6:15722–15723.
- Luo L, Zhang C, Zhao J, et al. Effects of rapamycin on reduction of peridural fibrosis: an experimental study. Med Sci Monit. 2015;21:482–488.
- Wang B, Ding W, Zhang M, et al. Rapamycin attenuates aldosterone-induced tubulointerstitial inflammation and fibrosis. Cell Physiol Biochem. 2015;35:116–125.
- Emile JF, Diamond EL, H´elias-Rodzewicz Z, et al. Recurrent RAS and PIK3CA mutations in Erdheim-Chester disease. Blood. 2014;124:3016–3019.
