Abstract
Background: Sex steroid hormones and their receptors are important in female sexual function. The aim of this study was to investigate the expression and distribution of estrogen receptor (ER)α, ERβ, G-protein-coupled ER-1 (GPER), androgen receptor (AR), progesterone receptor (PR)A, PRB and connective tissue growth factor (CTGF) in the vaginal wall among women who had been treated for cervical cancer with radiotherapy.
Material and methods: We included cervical cancer survivors treated with radiotherapy and premenopausal control women of the same age scheduled for benign gynecological surgery. We analyzed the expression and distribution of sex steroid hormone receptors and CTGF in biopsies from the vaginal wall, by real-time PCR and immunohistochemistry (IHC). Serum samples were analyzed for hormone levels and radiation dose at biopsy site were calculated and correlated to levels of the sex steroid hormone receptors.
Results: In the cervical cancer survivors (n = 34), we found a lower expression of ERα at both mRNA and protein levels, compared to the control women (n = 37). In the survivors with high radiation dose at biopsy site, the immunostaining of ERα and AR was lower in the epithelium and the stroma, compared to survivors with minimal radiation dose. The later group showed expression of ERα comparable to the control women. The cancer survivors were sufficiently substituted with systemic estradiol with no difference in the serum estradiol levels compared to control women.
Conclusions: We found that external radiation reduces the ERα and AR protein expression in the vaginal mucosa, indicating that the vaginal changes in irradiated cervical cancer survivors and the lack of response to hormonal treatment could be due to the decreases in sex steroid hormone receptor expression.
Introduction
Cervical cancer survivors have a high degree of late vaginal morbidity [Citation1]. The extensive treatment, often including radiotherapy, results in tissue changes. Vaginal atrophy and fibrosis develop over time, severely affecting quality of life and sexual function [Citation2,Citation3]. Estrogen therapy is often used in an attempt to diminish symptoms of dryness and dyspareunia, but in many cases the vaginal mucosa seems reluctant to respond. The presence of sex steroid hormone receptors in the vaginal mucosa is essential for effective hormone therapy in general. However, little is known about the expression of steroid hormone receptors in the vaginal wall in cervical cancer survivors treated with radiotherapy.
In women with postmenopausal vaginal atrophy without previous cervical cancer therapy, treatment with low-dose local vaginal estrogen therapy is effective and well tolerated [Citation4]. In a previous study, we found radiotherapy-induced morphological changes with fibrosis in the connective tissue of the vaginal wall in cervical cancer survivors [Citation5]. The vaginal morphological changes can affect the possible effectiveness of estrogen therapy, but it is unclear if the levels of intracellular estrogen receptors (ERs), which mediate the estrogen action, are affected.
The majority of new cases of cervical cancer are young or middle-aged women. Even in more advanced stages, when radiotherapy is used for cure, the prognosis is good and long-term survivors will live with the sequelae of the disease and treatment. For reduced symptoms and risk associated with early menopause, cervical cancer survivors are recommended systemic hormonal therapy up to the age of natural menopause [Citation6]. It is common that also local estrogen therapy is recommended, but clinically we do not observe the same response of the irradiated vaginal squamous epithelium as in postmenopausal atrophy [Citation7]. Data on vaginal side effects after radiotherapy are limited [Citation8–11]. To our knowledge, there is no previous study on steroid hormone receptor expression in the vaginal wall in cervical cancer survivors. We therefore, conducted a study to investigate the expression and distribution of ERα and β, G-protein-coupled ER-1 (GPER), androgen receptor (AR), progesterone receptor (PR)A, PRB and connective tissue growth factor (CTGF) in the vaginal wall among women who had been treated for cervical cancer with radiotherapy.
Material and methods
Subjects
We included women treated for cervical cancer with radiotherapy and control women of the same age. The cervical cancer survivors were treated at Karolinska University Hospital, Stockholm, between January 2004 and December 2007. They were treated with surgery and radiotherapy, often combined with concomitant Cisplatin, or primary chemoradiotherapy, according to regional guidelines at our institution at the time of the study. There are two articles with results from these subjects already published with focus on fibrotic and atrophic changes in the vaginal wall after radiotherapy [Citation5,Citation12]. A detailed description of the treatments has also previously been published [Citation5].
The inclusion criteria were diagnosis of cervical cancer and age <51 years at time of the study. The time from cancer treatment to study inclusion was between two to five years. Exclusion criteria were significant co-morbidity and recurrence of cervical cancer. We included women undergoing benign gynecological surgery at Danderyd Hospital, Stockholm, as controls. The control women were premenopausal and age <51 years. General exclusion criteria were history of cancer, current pregnancy and systemic diseases. A clinical examination excluded vulvovaginal infection and/or inflammatory lesions, which also were exclusion criteria. The Regional Research Ethics Committee approved the present study (EPN Stockholm Dnr 2003-753) and the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards, and all participants gave a written informed consent.
Data collection
Information on treatment modality was collected from the participants’ medical charts. A detailed description of the calculation of the radiation dose at the vaginal biopsy site has previously been published [Citation5]. We collected the prescribed field margins, target definition, set-up images, portal image films, treatment protocol and dose per fraction and the total prescribes dose for each patient. On portal image films, the vaginal introitus was identified. The distance from the lower border of the EBRT field was measured and the EBRT dose at the biopsy site was calculated. For the BT, we used orthogonal X-ray images and made an estimation of the delivered dose based on isodose-curves from the actual treatment for each patient. We calculated the EQD2 doses for both the EBRT and the BT, obtaining the total dose at the biopsy site. To analyze radiotherapy-induced levels of sex steroid hormone receptors, the radiation dose at the biopsy site was calculated in two different cancer treatment groups; (I) pre-operative brachytherapy (BT)+surgery and (II) surgery and radiation including external beam radiation therapy (EBRT) or primary chemoradiotherapy.
At a study visit, serum samples were taken and estradiol, progesterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), sex hormone-binding globulin (SHBG) and testosterone were determined by direct chemiluminescent immunometric assay (Immulite; Siemens, Munich, Germany). Free testosterone was determined by a formula using total testosterone, SHBG and a fixed concentration of albumin (40 g/l) [Citation13]. In the controls, without contraceptives, we defined the menstrual phases by the level of serum progesterone. The luteal phase was defined as serum progesterone >17 nmol/l, and the follicular phase for values below. From each participant two vaginal biopsies were taken, with a 3 mm forceps at the three and nine o’clock position, 3–4 cm from the vaginal introitus. One biopsy was preserved in RNAlater (Thermo Fisher Scientific Life Sciences, Waltham, MA) for further RNA isolation. The second biopsy was formalin-fixed and paraffin-embedded according to a standard protocol.
RNA preparation and reverse transcription
Total RNA from vaginal biopsies was purified with RNeasy® Mini Kit (Qiagen GmbH, Hilden, Germany) according to a procedure recommended by manufacturer, for RNA isolation from fibrous tissues, including a DNase treatment step. From each sample, 2 μg of total RNA was reverse transcribed at 37 °C for 60 min, in a final volume of 40 μl with a reaction mixture (Qiagen, Hilden, Germany) containing 1 × RT buffer, dNTP mix (0.5 mM each dNTP), 600 ng random primers (Invitrogen, Paisley, UK), 2 units RNase inhibitor (Qiagen, Hilden, Germany) and 4 units of Omniscript™ reverse transcriptase (Qiagen, Hilden, Germany).
Real-time PCR analysis
Real-time PCR was performed for ERα, ERβ, GPER, AR, PRAB, PRB and CTGF in an iCycler™ iQ Real Time PCR System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). For PCR, the cDNAs corresponding to 50–100 ng () RNA were added to 12.5 μl of iQ™ SYBR® Green Supermix (Bio-Rad, Hercules, CA, USA) and 0.3 μM of each oligonucleotide primer in a final volume of 25 μl. After initial incubation for 3 min at 95 °C, the samples were subjected to 40 cycles of 10 s at 95 °C, followed by 45 s annealing at 55–65 °C depending upon the genes (). All reactions were performed in duplicates. The purity of PCR products was confirmed by a melting curve analysis in all experiments (data not shown). The oligonucleotide primers for ERα, ERβ, GPER, AR, PRAB, PRB, CTGF and the housekeeping gene ribosomal protein P0 (RPLP0) are presented in , as well as their predicted sizes. All primers, except PRB, were designed to span an intron/exon boundary or to be on different exons, to eliminate amplification of contaminating DNA. The primers are based on the sequences of the human genes (see accession no. in ) and the primer pairs were designed or checked using the NCBI/Primer-BLAST program. Each PCR assay included a negative control containing an RNA sample without reverse transcription. It is not possible to design primers that will detect only PRA mRNA, since the mRNAs for PRA and PRB are transcribed from same gene. PRB mRNA is longer than PRA mRNA. Therefore, the primer pair for PRB mRNA will detect a part of the PRB mRNA that is unique and not translated into PRA, while the PRAB primers will detect both PRA and PRB mRNAs since they are directed to the common sequence of the mRNAs. To standardize the quantification method, RPLP0 was selected out of several tested housekeeping genes as an invariable internal control. The PCR amplification rate and the cycle threshold (Ct) values were related to a standard curve using iCycler iQ Optical System Software (Bio-Rad, Hercules, CA, USA). The values of relative expression of the genes of interest were normalized against the RPLP0 product.
Table 1. Clinical characteristics and treatments of cervical cancer survivors and control women.
Immunohistochemistry
The tissue sections were deparaffinized using xylene, rehydrated in graded ethanol and subjected to microwave treatment for antigen retrieval in 0.01 M sodium citrate buffer (pH 6.0) for 10 min and then allowed to cool for a further 20 min at room temperature (RT). Following washing in buffer (0.1 M PBS or TBS, pH 7.4), nonspecific endogenous peroxidase activity was blocked by treatment with 3% hydrogen peroxide (Merck, Darmstadt, Germany) in methanol for 10 min at RT. Following 10 min wash in buffer, sections were exposed for 30 min to normal horse or swine serum (Vector Laboratories, Burlingame, CA, USA) diluted in PBS or TBS in a humidified chamber at RT. The tissue sections were thereafter incubated with the primary antibody. The primary antibodies are presented in . Negative controls were prepared by replacing the primary antibody with the same concentration of the specie relevant IgG. All dilutions, buffers and incubation times for the different antibodies are shown in . After the incubation with horseradish peroxidase-avidin biotin complex (Vectastain Elite, Vector, Burlingame, CA, USA), the bound enzyme was visualized by the application of 3,3′-diaminobenzidine (DAKO Cytomation, Carpinteria, CA, USA). The sections were counterstained with hematoxylin and dehydrated before mounted with Pertex®.
Table 2. Hormone levels in cervical cancer survivors, divided in four different hormonal treatment groups, and control women divided in three different groups; follicular phase, luteal phase and controls with contraceptives.
Image analysis
We used Leica microscope connected to a computer with the Colorvision software (Leica Imaging System Ltd., Cambridge, UK) in order to assess the immunostaining quantitatively. In a systematic way, 10 fields were randomly selected from the squamous epithelium and the stroma, for quantification of the area of positively immunostained (brown) nuclei. The tissue types were analyzed separately. In a few samples, it was not possible to obtain 10 separate sites due to lack of representative tissue, in those samples all epithelium and/or stroma were measured. The total area of positively stained nuclei was measured and expressed as a ratio of the total area of cell nuclei (brown reaction product + blue hematoxylin). This method was used for analyzing ERα, ERβ, AR, PRA and PRB.
Manual scoring
GPER and CTGF immunostaining was assessed by manual scoring due to their mainly cytoplasmic localization. The scoring was performed by two independent observers (BM, LS) on a four-point scale from negative (0), (+) faint, (++) moderate and (+++) strong immunostaining. Comparing the results of this method, from two independent observers, shows good consistency between investigators [Citation14].
Statistical analysis
The main analyses were comparisons between cancer survivors and control women using the Mann–Whitney U test. Thereafter subgroup-analyses were performed comparing hormonal levels in cancer survivors with various hormonal treatments and controls in different phases of the menstrual cycle and those on oral contraceptives. Further subgroup-analyses were performed for the hormone receptor expression in the two treatment groups of cancer survivors. ANOVA on ranks (Kruskal–Wallis test) was used for comparing differences in more than two treatment groups and significances were evaluated by Dunn’s test, all pairwise multiple comparison procedures (Sigma-Plot 13, Alfasoft AB, Gothenburg, Sweden). According to our statistic consultant, the Bonferroni adjustments are appropriate when the study variables are independent. In our study, the variables are not independent, hormones affect other hormones and receptors, and therefore adjustments according to the Bonferroni will be too conservative and result in type-II errors, and are therefore not performed. Correlations were evaluated by Spearman’s test. Values were considered significantly different when p< .05.
Results
Thirty-four cervical cancer survivors and 37 control women met the inclusion criteria and agreed to participate in the study. The mean ages did not differ between the cervical cancer survivors and controls (). The cancer survivors’ oncological treatments are listed in . A more detailed description of the cancer treatments and FIGO stage has previously been published [Citation5]. Five patients were treated with preoperative BT only and received minimal radiation dose at biopsy site in the distal part of the vagina (treatment group I), median 0.0 Gy (range 0–9.0). The other 29 cancer survivors who were treated with EBRT and BT (treatment group II) received median 44.8 Gy (range 38.8–69) at the biopsy site. As a consequence of the radiotherapy, all the patients have ovarian failure, resulting in postmenopausal hormone levels. Systemic hormonal therapy was used by 79% of the patients. In 12% of the patients, when the cancer treatment did not include hysterectomy, estrogen therapy with 17β-estradiol was combined with gestagen (). Only 38% of the cervical cancer survivors used local estradiol therapy and the majority had started the treatment after symptoms of atrophy had occurred. Among the control women, 62% were not using hormonal contraceptive and they were divided into follicular and luteal phase according to the progesterone levels ().
Serum hormonal analyses
On a group level, there was no difference in the serum estradiol levels between all cancer survivors and control women (). The serum progesterone level was significantly lower among survivors, due to ovarian insufficiency after surgery and/or pelvic radiotherapy. The serum levels of FSH and LH were higher in the cancer survivors; i.e., postmenopausal values. Also SHBG was higher in the cancer survivors, but we found no differences in testosterone levels (). Cervical cancer survivors treated with both systemic and local estradiol had a higher serum level of estradiol than survivors treated with merely local estradiol and survivors with no hormonal treatment. There was no significant difference between the group of cancer survivors treated with only systemic estradiol and the other cancer survivors. Control women with contraceptives had lower estradiol levels compared to the cycling controls ().
In the subgroup analyses, we found no difference in the serum hormone levels between cancer survivors with minimal radiation dose at the biopsy site, treatment group I (n = 5), preoperatively BT only, compared to treatment group II, who had received a high radiation dose, EBRT + BT (n = 29). The serum estradiol levels showed median 108 pmol/l (interquartile range (IQR) (59.0–147.0) in treatment group I, compared to 145 pmol/l (85.0–208), p = .331, in treatment group II.
Real-time PCR analysis
In 26 out of the 34 biopsies from the cancer survivors and 29 out of the 37 biopsies from the control women, an adequate quality and quantity of RNA could be extracted. The relative mRNA expression (median and IQR) of ERα in the vaginal wall showed lower expression in the cancer survivors (0.5 (0.3–0.8)) compared to controls (0.8 (0.6–1.0), p = .007). The biopsies from the vaginal wall contained both the epithelium and the underlaying stroma. We found a higher mRNA expression of CTGF in the cancer survivors (0.6 (0.2–1.4)) compared to controls (0.2 (0.2–0.3), p = .002). No differences in the mRNA expression of steroid hormone receptors in the vaginal wall were found in the analyses between patients with minimal radiation dose at the biopsy site, compared to high radiation dose.
In the subgroup analyses, we saw a difference in the control group, with the highest relative expression of ERα mRNA in the follicular phase with median 0.93 (0.64–1.1), compared to the luteal phase 0.53 (0.45–0.77), p = .027. We found no differences in the subgroup analyses of the cancer survivors with different hormonal treatments.
Immunohistochemistry
Representative images from immunohistochemistry (IHC) and the scoring results in the epithelium and stroma are shown in and , respectively. In the cancer survivors, there were a lower immunostaining of ERα in the epithelium, but not in the stroma, compared to controls (). ERα positive cells were predominantly distributed along the basal membrane and less frequently in the stromal cells (; upper panel, left columns). The cancer survivors in the treatment group with a high radiation dose at the biopsy site, showed a lower immunostaining of ERα in the epithelium and the stroma compared to those who only received preoperative BT. The later group showed expression of ERα comparable to the control women (). The AR-expressing cells were found both in the epithelium and the stroma (; second panel, right columns). In the epithelium, there was a lower expression in the cancer survivors compared to controls, but no difference in the stroma ().
Figure 1. Representative images of immunostaining in the vaginal wall of cervical cancer survivors and controls. ERα: estrogen receptor α; ERβ: estrogen receptor β; GPER: G-protein coupled estrogen receptor 1; AR: androgen receptor; PRA: progesterone receptor A; PRB: progesterone receptor B; CTGF: connective tissue growth factor; epi: epithelium; str: stroma. Negative controls, where the primary antibody is replaced with the specie relevant IgG, are shown in the right bottom panel. The bar represents 100 µm.
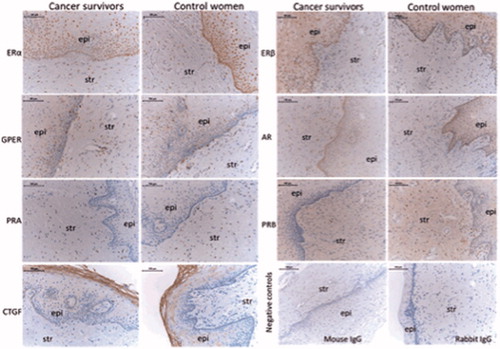
Table 3. Immunohistochemistry scores of steroid hormone receptors and CTGF in the vaginal epithelium and stroma of cervical cancer survivors and control women.
Women in the treatment group with external radiation had a lower AR-expression in both epithelium and stroma, compared to women with minimal radiation dose at the biopsy site (). The PRA- and PRB-expressing cells were sparsely distributed along the basal membrane in the epithelium and more frequently in the stroma, with a lower expression of PRB in the stroma among the cancer survivors (; third panel right columns). For the expression of GPER and CTGF, we found no differences between the two groups ().
We found no differences in the IHC scores of nuclear steroid hormone receptors in the vaginal epithelium and stroma in the analyses comparing cervical cancer survivors with different hormonal treatments (data not shown).
Correlation analysis
The correlation analyses were performed in the cancer survivors and control women separately. In the control women, we found a negative correlation between serum estradiol and ERα expression in the epithelium (R = –0.359, p = .032), but not in the stroma nor in the mRNA-expression of ERα. In the cancer survivors, there was a negative correlation between estradiol and the mRNA-expression of ERα (R = –0.459, p = .019), but no correlation on the protein level. Among control women, there was a positive correlation between estradiol and PRB-expression in the epithelium (R = 0.341, p = .042) and in the stroma (R = 0.400, p = .016). Estradiol correlated positively to the mRNA-expression of PRAB (R = 0.581, p = .001) in the controls. No correlations were found between estradiol and PRB/PRAB in the cancer survivors. We saw a positive correlation between testosterone and AR-expression in the epithelium (total testosterone (R = 0.367, p = .028), free testosterone (R = 0.378, p = .023)) in the control women, but not in the stroma nor in the mRNA-expression. No correlation between testosterone and AR-expression was found in the cancer survivors.
Discussion
Hormonal therapy with estrogen is recommended and widely used by cervical cancer survivors, but the therapeutic outcome in the vaginal wall is unclear. This is, to our knowledge, the first study on the steroid hormone receptor expression in the vaginal wall of cervical cancer survivors treated with radiotherapy. We found a lower expression of ERα, at both mRNA and protein levels, in cervical cancer survivors compared to control women. The survivors with high radiation dose at biopsy site showed lower expression of ERα in both epithelium and stroma, compared to survivors with minimal radiation dose. These results indicate that the vaginal mucosa in cervical cancer survivors treated with high dose radiotherapy has less potential to respond to estrogen treatment in the chronic phase.
Steroid hormone receptor expression is a dynamic process, normally affected by the serum steroid hormone levels. The treatment of advanced cervical cancer with radiotherapy results in complete cessation of the endogen estrogen production by the ovaries. We found that the cancer survivors and the controls had no difference in the serum estradiol on a group level, which implies that the survivors were sufficiently substituted. A majority had systemic estradiol therapy (79%), which can correspond to 62% of the controls that had regular menstruations and ovarian estradiol production. However, the cancer survivors with only local estradiol or no hormonal treatment at all showed significantly lower levels of estradiol. The level of SHBG was higher in the cancer survivors, as typically seen as a result of systemic oral estrogen therapy [Citation15]. If this affects the biologically active serum estradiol is not fully known.
The lower expressions of ERα in cervical cancer survivors were present at both mRNA and protein levels. In one previous study on ERα mRNA expression in the vaginal wall in pre- and postmenopausal women with vaginal prolapse, lower expression was found in the postmenopausal group, but no differences between the premenopausal women and the postmenopausal women treated with systemic estradiol [Citation16]. In other small studies, no differences were found in the ERα mRNA expression between pre- and postmenopausal women [Citation17,Citation18], but a lower ERβ mRNA expression in postmenopausal women [Citation17]. Comparing pre- and postmenopausal expression of the ERs in the vaginal wall using IHC has shown lower ERα expression in postmenopausal women compared to premenopausal. The expression increased in women treated with local estrogen [Citation19], less with systemic administration [Citation20]. These results demonstrate the well-known clinical effect of estradiol or estriol on the vaginal mucosa of the postmenopausal atrofic changes. The discrepans in the response to systemic treatment in postmenopausal women and the cancer survivors after radiotherapy, might be a result of fibrotic changes in the vaginal wall, affecting small vessels and thus omitting the hormone to reach the hormone receptors in the mucosa [Citation5].
Another interesting finding was that the survivors with high radiation dose at biopsy site showed lower expression of ERα in both epithelium and stroma, compared to survivors with minimal radiation dose. One may speculate if the radiation hampers the normal tissue response to estrogen therapy. To our knowledge, there are no studies evaluating modern hormonal therapy on vaginal changes in cervical cancer survivors treated with radiotherapy. In the only double-blind, placebo-controlled study on topical estrogen therapy after radiotherapy for cervical cancer, 0.01% dienestrol cream was used. In the evaluation with clinical examinations after 5–8 months, the results from the study showed less vaginal bleeding and the vaginal epithelium was considered normal in appearance in 43% compared to 10% in control women using cream without estrogen. Frequency of intercourse was equal in the groups, but the estrogen-treated group reported less dyspareunia [Citation9]. Dienestrol is a synthetic non-steroid estrogen with high binding affinity for ERα, two times as great compared to estradiol, the substance typically used in topical estrogen therapy today. Estriol, used in estrogen cream and gel in postmenopausal vaginal atrophy, has less affinity for ERα than estradiol. In one study on systemic estrogen therapy evaluating the effect on vaginal cytology in women treated with radiotherapy for cervical cancer, no benefits from treatment were seen. It must be pointed out though, that the women in the study were examined after just one week of systemic conjugated estrogen treatment [Citation10]. In our study, most cancer survivors started systemic estrogen therapy at the end of the oncological treatment, but regarding topical estrogen therapy the majority started after vaginal symptoms of atrophy had already occurred. Early start and accurate dosage, formula and type of local estrogen therapy might be of importance for normal tissue function, including preservation of receptor expression.
We found a lower expression of AR in the epithelium of cancer survivors compared to control women. In the subgroup analyses, AR expression was lower in the cancer survivors that had received a high radiation dose at the biopsy site. Higher AR protein and mRNA expression in the vaginal wall in premenopausal compared to postmenopausal women have been shown by Baldassarre et al. [Citation21]. Metabolic disorders, like type 2 diabetes, are associated with sexual dysfunction [Citation22]. In postmenopausal women with diabetes AR expression in the vaginal wall is reduced compared to postmenopausal women without diabetes [Citation23]. The AR is involved in regulation of vaginal hemodynamics and mucification [Citation24], and alterations in expression may contribute to changes in the vagina, affecting sexual response.
In animal studies, the expression of ERα was increased and PR was decreased in the vaginal wall in rats after ovariectomy, with loss of ovarian production of estradiol and progesterone [Citation25]. The same study showed that treatment with estradiol down-regulated ERα and up-regulated PR. In our control women, we saw the same dynamic interplay between serum estradiol and the negative correlation to ERα and positive correlation to PRB levels. There was also a positive correlation between testosterone and AR levels in the controls. None of these correlations were observed in the cancer survivors, which further indicate that the mucosa does not respond normally to sex steroid hormones after radiation. Our patients were examined between two to five years after completed radiotherapy, when the adverse side effect of the treatment is considered permanent. Again, the questions arise whether changes in recommendations for local estrogen treatment should be considered. The estrogen therapy used today is the same as for postmenopausal women with a responsive mucosa. After radiation, an early start, higher doses and/or increased frequency might preserve the mucosal ability to respond to estrogen.
We found a higher mRNA expression of CTGF in the vaginal wall in the cancer survivors compared to controls. CTGF is a central mediator of tissue remodeling and fibrosis [Citation26]. We have earlier shown that cervical cancer survivors have radiotherapy-induced fibrosis and elastosis in the connective tissue of the vaginal wall [Citation5]. This result supports our previous finding.
The strengths of this study are that we analyzed the steroid hormone expression at both mRNA and protein levels, the standardized sampling and the inclusion criteria of women with no age-induced vaginal changes. However, there are several limitations of the study. The heterogenous material, concerning hormonal status in both cancer survivors and in control women, might affect the result of mRNA and receptor expression. We have tried to avoid this problem by performing subgroup analyses, but the interpretation of the results could be doubtful due to small sample-sizes. The material, with the heterogenous hormonal treatment regimens being used, probably reflects the clinical variability and variation in compliance once the cervical cancer treatment is completed. Another limitation could be that correction for multiple analyses was not performed. However, the hormones and the receptor levels are not independent variables, since they affect each other. Performing a Bonferroni for multiple comparisons would be too conservative and result in an increase of type II errors.
In conclusion, we found that external radiation reduces the ERα and AR expression in the vaginal mucosa in cervical cancer survivors. Our results indicate that the vaginal changes in irradiated cervical cancer survivors and the lack of response to hormonal treatment could partly be due to the decrease in sex steroid hormone receptor expression.
Supplemental Material
Download PDF (106.6 KB)Acknowledgments
The authors are grateful to Elisabeth Bergh, Unit for Medical statistics (MedStat), Department of Learning, Informatics, Management and Ethics, Karolinska Institutet, for statistical expertise.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Ye S, Yang J, Cao D, et al. A systematic review of quality of life and sexual function of patients with cervical cancer after treatment. Int J Gynecol Cancer. 2014;24:1146–1157.
- Bergmark K, Avall-Lundqvist E, Dickman PW, et al. Vaginal changes and sexuality in women with a history of cervical cancer. N Engl J Med. 1999;340:1383–1389.
- Jensen PT, Groenvold M, Klee MC, et al. Longitudinal study of sexual function and vaginal changes after radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2003;56:937–949.
- North American Menopause S. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14:355–369, quiz 70–1.
- Hofsjo A, Bohm-Starke N, Blomgren B, et al. Radiotherapy-induced vaginal fibrosis in cervical cancer survivors. Acta Oncol. 2017;56:661–666.
- Pitkin J, Rees MC, Gray S, et al. Management of premature menopause. Menopause Int. 2007;13:44–45.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016;31:CD001500.
- Pitkin RM, Bradbury JT. The effect of topical estrogen on irradiated vaginal epithelium. Am J Obstet Gynecol. 1965;92:175–182.
- Pitkin RM, VanVoorhis LW. Postirradiation vaginitis. An evaluation of prophylaxis with topical estrogen. Radiology. 1971;99:417–421.
- Abitbol MM, Davenport JH. The irradiated vagina. Obstet Gynecol. 1974;44:249–256.
- Denton AS, Maher EJ. Interventions for the physical aspects of sexual dysfunction in women following pelvic radiotherapy. Cochrane Database Syst Rev. 2003;1:CD003750.
- Hofsjo A, Bergmark K, Blomgren B, et al. Radiotherapy for cervical cancer – impact on the vaginal epithelium and sexual function. Acta Oncol. 2018;57:338–345.
- Sodergard R, Backstrom T, Shanbhag V, et al. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810.
- Isaksson E, Wang H, Sahlin L, et al. Effects of long-term HRT and tamoxifen on the expression of progesterone receptors A and B in breast tissue from surgically postmenopausal cynomolgus macaques. Breast Cancer Res Treat. 2003;79:233–239.
- Stomati M, Hartmann B, Spinetti A, et al. Effects of hormonal replacement therapy on plasma sex hormone-binding globulin, androgen and insulin-like growth factor-1 levels in postmenopausal women. J Endocrinol Invest. 1996;19:535–541.
- Gebhart JB, Rickard DJ, Barrett TJ, et al. Expression of estrogen receptor isoforms alpha and beta messenger RNA in vaginal tissue of premenopausal and postmenopausal women. Am J Obstet Gynecol. 2001;185:1325–1330; discussion 30–31.
- Soderberg MW, Johansson B, Masironi B, et al. Pelvic floor sex steroid hormone receptors, distribution and expression in pre- and postmenopausal stress urinary incontinent women. Acta Obstet Gynecol Scand. 2007;86:1377–1384.
- Chen GD, Oliver RH, Leung BS, et al. Estrogen receptor alpha and beta expression in the vaginal walls and uterosacral ligaments of premenopausal and postmenopausal women. Fertil Steril. 1999;71:1099–1102.
- Fuermetz A, Schoenfeld M, Ennemoser S, et al. Change of steroid receptor expression in the posterior vaginal wall after local estrogen therapy. Eur J Obstet Gynecol Reprod Biol. 2015;187:45–50.
- Sawczuk B, Gołębiewska M, Mazurek A, et al. Immunohistochemical evaluation of oestrogen receptors alpha and beta in epithelium of the vaginal mucous membrane in women after oestrogen therapy. PM. 2017;1:12–18.
- Baldassarre M, Perrone AM, Giannone FA, et al. Androgen receptor expression in the human vagina under different physiological and treatment conditions. Int J Impot Res. 2013;25:7–11.
- Kizilay F, Gali HE, Serefoglu EC. Diabetes and sexuality. Sex Med Rev. 2017;5:45–51.
- Baldassarre M, Alvisi S, Berra M, et al. Changes in vaginal physiology of menopausal women with type 2 diabetes. J Sex Med. 2015;12:1346–1355.
- Traish AM, Kim NN, Huang YH, et al. Sex steroid hormones differentially regulate nitric oxide synthase and arginase activities in the proximal and distal rabbit vagina. Int J Impot Res. 2003;15:397–404.
- Pessina MA, Hoyt RF, Jr., Goldstein I, et al. Differential regulation of the expression of estrogen, progesterone, and androgen receptors by sex steroid hormones in the vagina: immunohistochemical studies. J Sex Med. 2006;3:804–814.
- Lipson KE, Wong C, Teng Y, et al. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5:S24.
