Abstract
Introduction: Adjuvant whole-pelvic radiation therapy (WPRT) improves locoregional control for high-intermediate stages I–III endometrial cancer patients. Intensity modulated radiation therapy (IMRT) tends to replace the standard 3D conformal radiation therapy (3DCRT) technique used in trials.
Material and methods: Consecutive patients with stages I–IIIc endometrial cancer treated between 2008 and 2014 in our department with post-operative 3DCRT or IMRT WPRT were studied retrospectively. Patients with cervical involvement underwent additional low-dose rate vaginal brachytherapy. The impact of the WPRT technique on local control, tolerance, disease-free survival (DFS) and overall survival (OS) was assessed. Clinicians evaluated routinely acute radiation toxicity each week during radiation therapy and late toxicity during standard follow-up consultations.
Results: Median follow-up was 50 months (range: 6–158). Among the 83 patients included, 47 were treated with 3DCRT and 36 with IMRT. There was no difference in patient characteristics between groups. The 5-year locoregional control and DFS rates were 94.5% and 68%, respectively. No significant difference was found between the 3DCRT and IMRT groups in terms of survival, with 5-year OS rates of 74.6% and 78%, respectively. In multivariate analysis, age over 68, stage > T1 and grade 3 were independently associated with shorter DFS and OS. Seven patients (8.4%) had grades 3–4 acute gastrointestinal (GI) toxicity with five patients (10.6%) and two (5.4%) in the 3DCRT and IMRT groups, respectively (p = .69). One case (1.2%) of late grade 3 GI toxicity was observed treated in 3DCRT.
Conclusions: IMRT seems to be a safe technique for the treatment of endometrial cancer with a trend towards decreased acute GI toxicities. Results of the phase 3 RTOG 1203 trial are needed to confirm these results.
Introduction
According to the Global Cancer statistics for 2018, with 382,069 new cases diagnosed yearly and 89,929 deaths worldwide, endometrial cancer is a frequent and serious disease, especially in Europe and North America due to the impact of obesity on its physiopathology [Citation1].
After hysterectomy, whole-pelvic radiation therapy (WPRT) is recommended for high-intermediate and high-risk stage I, stage II, and III endometrial cancer [Citation2]. Several studies have shown a reduction in locoregional recurrence and a good tolerance [Citation3–6]. In the PORTEC 1 trial, the 15-year actuarial locoregional recurrence rates were 6% in the group treated by WPRT versus 15.5% in the group treated with surgery alone (p < .0001) [Citation3,Citation7]. In the meta-analysis by Kong et al., WPRT has permitted a reduction in locoregional relapse, with a relative risk (RR) of 0.28 (95% confidence interval [95% CI] = 0.17–0.44) [Citation4].
Considering WPRT has not been shown to have an impact on overall survival (OS), other adjuvant modalities have been investigated. The PORTEC 2 trial showed that vaginal brachytherapy alone could be an alternative for high intermediate risk endometrial cancer. However, the pelvic recurrence was significantly higher in the vaginal brachytherapy alone arm (3.8%) than in the pelvic irradiation arm (0.5%) (p = .02) [Citation8]. Another option is adjuvant chemotherapy associated to radiation therapy. However, in a recent randomized trial, de Boer et al. reported that adjuvant chemotherapy given during and after radiotherapy for high-risk endometrial cancer did not improve 5-year OS [Citation9].
Since the beginning of the 2000s, intensity modulated radiation therapy (IMRT) tends to replace the standard 3D conformal radiation therapy (3DCRT) technique that has been widely used for the treatment of pelvic tumors. Integrating advances in engineering (multi-leaf collimators) and informatics (inverse dosimetric planning), this modern technique of external beam radiation therapy allows the delivery of a highly conformal treatment without compromising the target volume coverage. Hence, IMRT has become a common strategy for WPRT, and has been shown to reduce the dose received by surrounding organs at risk (OARs) [Citation10,Citation11]. Therefore, using IMRT should in theory improve the tolerance of the treatment without compromising its efficacy. A recent Cochrane Database systematic review supports these assumptions, finding IMRT significantly better than 3DCRT in terms of gastrointestinal (GI) toxicity in the context of pelvic irradiation but with limited data on local control and survival [Citation12].
In this context, the outcomes of patients with endometrial cancer treated with post-operative WPRT using 3DCRT or IMRT were retrospectively studied and the impact of these two techniques on local control, tolerance and survival was assessed.
Patients and methods
Patients
All consecutive patients treated in the department for endometrial cancer by WPRT from 2008 to 2014 were reviewed. Patients with histologically proven high-intermediate and high-risk stages I–IIIC endometrial cancer according to the ESMO-ESGO-ESTRO (European Society for Medical Oncology - European Society of Gynaecological Oncology - European Society for Radiotherapy and Oncology) classification with no distant metastasis at diagnosis were eligible for inclusion [Citation2]. Therapeutic management was decided on by a multidisciplinary tumor board on an individual basis, according to the French National Institute of Cancer guidelines [Citation13]. Prior to WPRT, all patients had undergone total hysterectomy and bilateral salpingo-oophorectomy with or without lymphadenectomy. We included patients with a type 1 (endometrioid) or type 2 (serous, clear cell or carcinosarcoma) endometrial cancer.
Treatment
For computed tomography (CT) simulation, a CT scan was acquired in a supine position with a full bladder and an empty rectum. Two series were acquired: one without injection of intravenous contrast for dose calculation, and a second with contrast to help delineation. The upper limit of the acquisition was the L2–L3 interspace and the lower limit was 2 cm below the lesser trochanter. We applied the same delineation protocol for both radiation therapy techniques. The clinical target volume (CTV) included the upper third of the vagina, vaginal vault, regional lymph nodes (common iliac, internal and external iliac, presacral areas) with vessels and paranodal tissues. The planning tumor volume (PTV) was defined by adding a 1-cm margin around the upper third of the vagina and a 5-mm margin around the nodal CTV. Stage IIIC2 patients also received radiation to the para-aortic region.
OARs included the entire bladder, anal canal, rectum, bowel (delineated as the peritoneal cavity until 2 cm above the PTV) and femoral heads. For patients treated using IMRT, iliac bones, pubic symphysis and vulva were delineated.
According to the International Commission on Radiation Units and Measurements (ICRU) 83 recommendations, treatment planning aims were that 95% of the PTV received at least 95% of the prescribed dose with a maximum dose not exceeding 107% of the latter [Citation14]. For optimization and approval, we used the same dose constraints for the OARs in 3DCRT and IMRT, except for in 3DCRT for the dose to the bowel bag (dose reporting) and the iliac bone (not evaluated) (Supplemental Table 1).
3DCRT was performed with anterior, posterior and two lateral beams. IMRT was performed with five to ten beams with a step and shoot technique. All patients had daily megavoltage imaging to control their positioning before treatment.
When the cervix was infiltrated, postoperative low-dose rate (LDR) vaginal brachytherapy was administered after WPRT using the vaginal mold technique with after loaded 192Ir sources. This technique was described in previous studies [Citation15,Citation16]. The prescription at the reference isodose, defined at 5 mm from the applicator, was 15 Gy.
According to the ESMO recommendations, patients with histological type II, FIGO stage III (International Federation of Gynecology and Obstetrics), lymphovascular space invasion (LVSI) received adjuvant chemotherapy if indicated. Usually, it consisted in six cycles of carboplatin AUC 5 and paclitaxel 175 mg/m2 every 3 weeks administered between surgery and WPRT.
Endpoints
The primary endpoint was locoregional relapse, defined as a relapse of the primary tumor at the vaginal vault or pelvic lymph nodes, suggesting in-field failure [Citation17,Citation18]. It was calculated from the time of diagnosis to the date of any evidence of locoregional recurrence or last follow-up.
Secondary endpoints were acute and late toxicity, OS, and disease free survival (DFS). OS was defined as the time from the date of diagnosis to the date of death from any cause or last follow-up. DFS was measured from the date of diagnosis to the date of any evidence of local recurrence, distant metastasis or last follow-up.
Toxicity assessment and follow-up
We studied the most frequent toxicities, such as GI and genitourinary (GU). Acute and late toxicities were defined as an event occurring within and after 90 days from the start of WPRT, respectively.
Clinicians graded routinely acute GI and GU radiation toxicity each week during radiation therapy, according to the common terminology criteria for adverse events (CTCAE) version 4.0 scale.
Follow-up evaluation with pelvic examination, thoraco-abdomino-pelvic CT, ACE, and CA 125 dosages was performed at 1-month post-WPRT, and then every 3 months for 6 months, every 6 months for 4.5 years and annually thereafter. Late toxicity was scored according to LENT-SOMA (Late effects of normal tissues - Subjective Objective Management Analytic) scale during each follow-up consultation.
Statistical analysis
The impact on outcomes of radiation therapy technique and usual predictive factors identified in the literature were evaluated in univariate and multivariate analysis.
Baseline characteristics between groups were compared using the chi-square and Fisher’s exact tests. The cumulative probabilities of OS and DFS rates were calculated using the Kaplan–Meier method and compared using a log rank test. Univariate and multivariate analyses were done using Cox’s regression. For multivariate analyses, factors with a p < .2 in univariate analysis were included. For comparison of dosimetric data a Student’s t-test was performed with a one-sided superiority test. A p-value less than .05 was considered statistically significant. All statistical analyses were performed using R Studio software (version 3.3.2), RStudio Inc.
The institutional review board of the institution approved this monocentric retrospective study.
Results
We retrospectively reviewed the medical files of 96 consecutive women treated for endometrial cancer with WPRT from December 2008 to December 2014 in the department. Thirteen patients were excluded: eight had metastatic disease at diagnosis, four had undifferentiated sarcoma and the last had not been operated. Finally, the study population was composed of 83 patients treated with post-operative WPRT. Characteristics of the 83 patients are reported in .
Table 1. Patient characteristics.
Most of the women were menopausal (97.6%) with a median age of 68 years (range: 40–86). They mostly had a myometrial invasion superior to 50% (66.3%) and no vaginal extension (90.4%). FIGO stage III (54.2%) and grades 1–2 (59%) cancers were the most represented in the population.
Forty-seven patients (56.6%) received adjuvant chemotherapy combining paclitaxel and carboplatin for the large majority (95.6%) with a median of 5.4 cycles (range: 2–6).
Regarding the irradiation technique, 47 patients were treated by 3DCRT between December 2008 and April 2012. Since the implementation of IMRT in the department in May 2012, 36 patients were treated by IMRT between June 2012 and December 2014.
There was no difference between the 3DCRT and IMRT groups regarding age, histology type, grade, FIGO stage, LVSI status, adjuvant chemotherapy and brachytherapy ().
The median dose was 45 Gy (range: 41.4–55 Gy) in 20 fractions and 35 days (range: 21–59 days). One patient had to stop WPRT after 21 days because of a grade 3 GI toxicity in the IMRT group but was kept in the study for the statistical analyses. Six patients received a lymph node boost with a median dose of 8.9 Gy (4.5–10). Twenty-four patients (29%) underwent LDR vaginal brachytherapy after WPRT because of cervical involvement, 11 in the 3DCRT group (30.5%) and 13 in the IMRT group (27.7%), p = .96. The median interval between WPRT and vaginal brachytherapy was 19 days (range: 5–48). The median total dose at the reference isodose was 15 Gy (range: 10–20 Gy). The median dose at the ICRU rectal point was 11.3 Gy (range: 4–25 Gy), and the median dose at the ICRU bladder point was 7.85 Gy (range: 2.4–15.9 Gy).
Median follow-up was 50 months (range: 6–158 months). During follow-up, five locoregional relapses were observed: two nodal (iliac) and two local relapses (vaginal and sigmoid) in the 3DCRT group and one nodal iliac relapse in the IMRT group. The locoregional control rate was 96.2% (95% CI = 92.1–100) and 94.5% (95% CI = 89.3–100) at 2 and 5 years of follow-up, respectively. In univariate analysis, we did not find any statistically significant factor related to an increased risk of locoregional relapse.
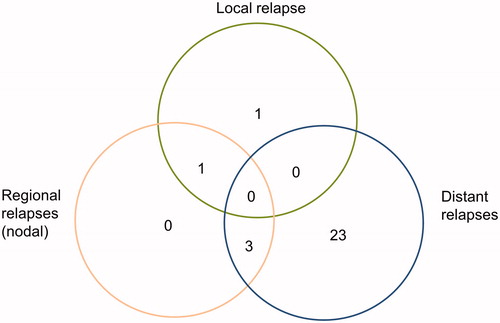
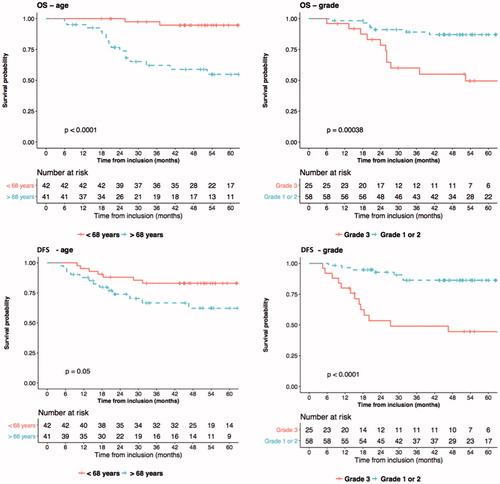
Twenty-eight relapses were observed: all locoregional relapses were associated with distant nodal metastasis, except one (). The most frequent locations of distant relapses were peritoneal carcinomatosis (eight patients) and lung metastasis (eight patients). Twenty distant relapses occurred in patients treated by 3DCRT and eight in patients treated by IMRT (p = .005). The 5-year DFS rate was 60.3% (95% CI = 47.6–76.4) in the 3DCRT group, and 76.2% (95% CI = 63–92.2) in the IMRT group (p = .5). In the univariate analysis, four factors were statistically related to an increased risk of tumor relapse: age over 68 years, grade 3, the absence of lymphadenectomy and a trend for T stage > T1 (, ). In the multivariate analysis, three factors were independently significant: age over 68 years, stage > T1 and grade 3 ().
Table 2. Predictive factors of any tumor relapse.
During the follow-up period, twenty deaths occurred: thirteen (27.7%) and seven (19%) patients died in the 3DCRT and IMRT groups, respectively. The 5-year OS was 74.6% (range: 63–89%) and 78% (range: 65–94%) in the 3DCRT and IMRT group, respectively (p = .8). In the univariate analysis, three statistically significant factors predicted a worse OS: age over 68 years, grade 3 and the absence of lymphadenectomy (, Supplemental Table 2). In the multivariate analysis, three factors were independently significant: age over 68 years, stage > T1 and grade 3 (Supplemental Table 2).
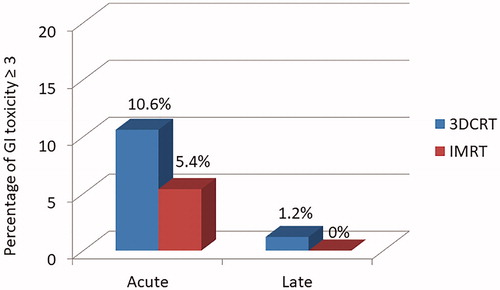
Fifty-three patients presented acute GI toxicity of any grade (64%). Sixteen patients (34%) and 11 patients (30.5%) had at least one grade 2 toxicity in the 3DCRT and IMRT groups respectively (p = .7). Five (10.6%) patients and two patients (5.4%) had grade 3 GI toxicity in the 3DCRT and IMRT groups, respectively (p = .69; ). Among patients treated in IMRT who had at least a grade 2 GI toxicity, three patients underwent brachytherapy, one received chemotherapy and five had a body mass index < 29 kg/m2. No grade 4 or 5 toxicity was observed in either group. Thirty patients had acute GU toxicity of any grade (36%). Seven patients (15%) and one patient (2.7%) had grade 2 toxicity in the 3DCRT and IMRT groups, respectively (p = .1). No grade ≥3 GU toxicity was observed in both groups.
Figure 3. Subset analysis of disease free survival and overall survival for patients according to age and histological grade.
A late GI toxicity event was reported in one patient: a radiation enteritis of grade 3. The patient was treated with 3DCRT and underwent surgery for radiation enteritis 2.5 years after the end of WPRT. Three patients (3.6%) had chronic grade 1 GU toxicity: one patient was treated with 3DCRT and two with IMRT (p = .58). No case of grade ≥2 chronic GU toxicity was observed in any group. However, significant differences, with a lower mean D2% of bladder and rectum, and for the mean bladder V40 were found in favor of the IMRT group ().
Table 3. Dosimetric results for the OARs bladder and rectum.
Among the factors previously mentioned, no prognostic determinant factor of acute or late toxicity were found.
Discussion
In agreement with previous studies, in our experience IMRT was not inferior to 3DCRT in terms of locoregional control, DFS and OS in the adjuvant treatment of high-intermediate and high-risk endometrial cancer [Citation19–24]. This study also seemed to find less early GU and early and late GI toxicities after IMRT compared to 3DCRT.
The 5-year locoregional control rate was 96% with no significant difference between the two groups, similar to the rates reported in the ASTEC (A Study in the Treatment of Endometrial Cancer) trial and EN.5 meta-analysis [Citation20].
The role of lymphadenectomy in endometrial control remains controversial. Herrera et al. found that lymphadenectomy was a prognostic factor of locoregional control [Citation21]. A recent update of a Cochrane meta-analysis including two randomized control studies, found that no randomized control trial showed an impact of lymphadenectomy on DFS or OS [Citation25]. In our study, there was a trend towards lymphadenectomy as a prognostic factor of locoregional control but also in OS in univariate analysis, which has not been found in previous studies.
Endometrial cancer is mostly diagnosed at an early stage with a good prognosis and high survival rates. Hence, tolerance of therapies and quality of life are a particular concern for these patients [Citation26–28].
In our experience, a lower incidence of GI toxicity was noted in the IMRT compared to the 3DCRT group. Indeed, our results were not significant, which may be explained by the sample size and the low number of events. However, we observed a rough decrease of 50% between the two groups: 10.6% and 5.4% of patients exhibited acute grade 3 GI toxicity in the 3DCRT and IMRT groups, respectively (p = .69). When comparing the average dosimetric results for the bladder and rectum per group, there was a significant advantage in favor of a lower D2% for bladder and rectum in the IMRT group (p = .08; p = .03) compared to 3DCRT group, as well as a lower V40 for the bladder in the IMRT group (p < .001). This study is one of the largest retrospective studies reporting toxicity with 3DCRT and IMRT in endometrial cancer and our results are in agreement with Chen et al. who reported 6.2% of grade ≥3 acute GI toxicity after IMRT [Citation29]. Mundt et al. have published two retrospective studies including, respectively, 15 and 36 patients with gynecological tumors treated with IMRT showing a significant reduction in acute GI toxicity and chronic toxicities [Citation29–31]. Beriwal et al., in a series of 47 patients with endometrial cancer treated by adjuvant WPRT and HDR (High Dose Rate) brachytherapy, reported only one case of grade 3 GI toxicity [Citation19]. Similarly, in a series of 46 patients with high risk endometrial cancer treated with postoperative WPRT, Shih et al. [Citation20] reported only two cases of grade 3 toxicity, one acute and one chronic. In the RTOG 0418 phase II trial, 43 patients with stages IB–IIIC endometrial cancers have been included. Among them, 28% presented acute GI toxicities including nine grade 2 and three grade 3 [Citation21].
The impact of IMRT on toxicity is currently investigated in the RTOG 1203 phase III study, in which 278 patients with endometrial or uterine cervical cancer are randomized between a four-field-standard 3DCRT and IMRT. Preliminary results showed a decrease in toxicity and a better quality of life after 5 weeks of irradiation with IMRT [Citation27]. In this study, 30% of the patients treated with IMRT presented at least a grade 2 GI toxicity with 5.4% presenting a grade 3 GI toxicity. This is consistent with the preliminary results of the French prospective multi-institutional clinical trial RTCMIENDOMETRE designed to evaluate the incidence of toxicity after IMRT at a dose of 45 Gy in patients with FIGO IB-II stage endometrioid cancers with 27% of grade ≥2 acute GI toxicity [Citation23]. These results are in agreement with our current study, reporting 30.5% of GI toxicity after IMRT including 23.4% of grade ≥2. Conversely, Cho et al. have recently conducted a prospective trial with 120 patients with cervical or endometrial malignancies showing no difference in acute GI and GU toxicities between 3DCRT and IMRT, but the median dose was 5 Gy higher in IMRT group [Citation32]. Finally, a recent review of the Cochrane database demonstrated that WPRT treated in IMRT may reduce acute toxicities versus 3DCRT (RR = 0.48, 95% CI = 0.26–0.88) and at least grade 2 late GI toxicity (RR = 0.37, 95% CI = 0.21–0.65), but with a low-certainty evidence. The authors included only two trials about prostate and cervical cancers but no endometrial cancer [Citation12].
Considering long-term toxicities, we reported only one case of grade 3 radiation enteritis, in the 3DCRT group. Foerster et al. reported a significant decrease in chronic intestinal symptoms using IMRT [Citation33]. In the present study, probably due to the low number of events and patients older than 80 years old, no predictive factor of toxicity was found while Rovirosa et al. found an increase in severe late small bowel toxicity after IMRT in patients ≥80 years [Citation34].
In contrast to GI toxicity, little data are available on GU toxicity. In the present study, 30 patients had acute GU toxicity of any grade (36%). Again, although our results were not significant, possibly due to the small sample size and the low number of events, the rate of GU grade 2 toxicity roughly decreased by over 10%, with 15% and 2.7% in the 3DRCT and IRMT groups, respectively (p = .1). Our results contrast with those of a previous study reporting no severe GU toxicity after IMRT [Citation29]. In the RTCMIENDOMETRE trial, the rate of grade 2 GU toxicity was 19%, while only 2.7% have been noted in our study. This apparent discrepancy in the rate of GU toxicity can be partly explained by the rate of brachytherapy well known to increase local toxicities of external beam radiation therapy (75% in RTCMIENDOMETRE versus 29% in our study) [Citation8]. Finally, Soisson et al. showed that endometrial cancer survivors were at elevated risk for urinary system disorders between 1 and 5 years (RR = 1.64, 95% CI = 1.50–1.78). They also underlined that patients with higher stage, higher grade, older age and treated with radiation therapy or chemotherapy were at higher risk for urinary disorders [Citation35]. From the physiopathological point of view, the decrease in both GI and GU toxicity can be explained by the IMRT technique. Indeed, Heron et al. showed a significant reduction of the volume receiving 30 Gy (V30) with IMRT by about 66%, 52% and 36%, in rectum, bowel and bladder, respectively [Citation36].
When considering the secondary endpoints, in the IMRT group, 5-year DFS and OS were 76.2% and 78%, respectively, which is consistent with the literature [Citation22]. In our multivariate analysis, we confirmed that age over 68 years, stage > T1 and grade 3 were independent factors for both DFS and OS [Citation21,Citation23] while LVSI status was not significant.
Some limitations must be considered for this study. First, the retrospective nature as well as the sample size cannot exclude bias. However, the consistency of treatment planning and administration in the department as well as the delineation technique, treatment machine and the verification of patient positioning remained constant during the period study contribute to limit the risk of bias. Second, the absence of stratification according to tumor size is another limit as previous studies reported a higher risk of recurrence for patients with a tumor size superior to 3 cm [Citation37,Citation38]. Third, late toxicity should be analyzed with caution as the follow-up of patients treated by IMRT is lower than that of 3DRCT group. However, no difference in survival was noted between the groups. The number of tumor relapses significantly different may be explained by the difference of follow-up between the groups.
Despite the limits of the present study, our results support that patients treated with whole pelvis IMRT had no difference in tumor control and survival, with a decreased toxicity compared to those treated with 3DCRT. This study is one of the biggest retrospective study about toxicity between 3DCRT and IMRT in endometrial cancer. These findings must be interpreted with caution due to the monocentric and retrospective nature of this study. We await the results of the prospective phase III RTOG1203 trial, closed to inclusions, which may confirm ours.
Supplemental Material
Download MS Word (40.3 KB)Disclosure statement
The authors declare no conflict of interest.
References
- Bray F, Ferlay J, Soerjomataram I, et al. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68:1–31.
- Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Radiother Oncol. 2015;117:559–581.
- Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post operative radiation therapy in endometrial carcinoma. Lancet (London, England). 2000;355:1404–1411.
- Kong A, Johnson N, Kitchener HC, et al. Adjuvant radiotherapy for stage I endometrial cancer: an updated Cochrane systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:1625–1634.
- Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a gynecologic oncology group study. Gynecol Oncol. 2004;92:744–751.
- Klopp AH, Jhingran A, Ramondetta L, et al. Node-positive adenocarcinoma of the endometrium: outcome and patterns of recurrence with and without external beam irradiation. Gynecol Oncol. 2009;115:6–11.
- Creutzberg CL, Nout RA, Lybeert MLM, et al. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:631–638.
- Nout RA, Smit V, Putter H, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet 2010;375:816–823.
- de Boer SM, Powell ME, Mileshkin L, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;2045:1–15.
- Jouglar E, Barillot I. Référentiels et techniques d’irradiation de haute précision: cancer de l’endomètre. Cancer/Radiotherapie 2014;18:495–500.
- Yang R, Xu S, Jiang W, et al. Dosimetric comparison of postoperative whole pelvic radiotherapy for endometrial cancer using three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, and helical tomotherapy. Acta Oncol (Madr). 2010;49:230–236.
- Lawrie TA, Green JT, Beresford M, et al. Interventions to reduce acute and late adverse gastrointestinal effects of pelvic radiotherapy. Cochrane Database Syst Rev. 2017;1:CD012529.
- Professionnelles Recommandations. ©Cancer de l’endomètre Collection, Recommandations & référentiels, INCa, Boulogne-Billancourt, November 2010.
- Grégoire V, Mackie TR. State of the art on dose prescription, reporting and recording in intensity-modulated radiation therapy (ICRU report No. 83). Cancer/Radiotherapie. 2011;15:555–559.
- Huguet F, Cojocariu OM, Levy P, et al. Preoperative concurrent radiation therapy and chemotherapy for bulky stage IB2, IIA, and IIB carcinoma of the uterine cervix with proximal parametrial invasion. Int J Radiat Oncol Biol Phys. 2008;72:1508–1515.
- Chassagne DP. La plésiocuriethérapie des cancers du vagin par moulage plastique avec l’iridium 192 (préparation non radioactive). J Radiol Electrol Med Nucl. 1966;47:89–93.
- Creutzberg CL, Van Putten WLJ, Koper PC, et al. Survival after relapse in patients with endometrial cancer: results from a randomized trial. Gynecol Oncol. 2003;89:201–209.
- Sorbe BG, Horvath G, Andersson H, et al. External pelvic and vaginal irradiation versus vaginal irradiation alone as postoperative therapy in medium-risk endometrial carcinoma: a prospective, randomized study - quality-of-life analysis. Int J Gynecol Cancer. 2012;22:1281–1288.
- Beriwal S, Jain SK, Heron DE, et al. Clinical outcome with adjuvant treatment of endometrial carcinoma using intensity-modulated radiation therapy. Gynecol Oncol. 2006;102:195–199.
- Shih KK, Milgrom SA, Abu-Rustum NR, et al. Postoperative pelvic intensity-modulated radiotherapy in high risk endometrial cancer. Gynecol Oncol. 2013;128:535–539.
- Jhingran A, Winter K, Portelance L, et al. A phase II study of intensity modulated radiation therapy to the pelvis for postoperative patients with endometrial carcinoma: radiation therapy oncology group trial 0418. Int J Radiat Oncol Biol Phys. 2012;84:e23–e28.
- He S, Gill BS, Heron DE, et al. Long-term outcomes using adjuvant pelvic intensity modulated radiation therapy (IMRT) for endometrial carcinoma. Pract Radiat Oncol. 2017;7:19–25.
- Barillot I, Tavernier E, Peignaux K, et al. Impact of post operative intensity modulated radiotherapy on acute gastro-intestinal toxicity for patients with endometrial cancer: results of the phase II RTCMIENDOMETRE French multicentre trial. Radiother Oncol. 2014;111:138–143.
- Bouchard M, Nadeau S, Gingras L, et al. Clinical outcome of adjuvant treatment of endometrial cancer using aperture-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;71:1343–1350.
- Frost J, Webster K, Morrison J. Lymphadenectomy for the management of endometrial cancer (review) summary of findings for the main comparison. Cochrane Database Syst Rev. 2017;9:CD007585.
- Klee M, Machin D. Health-related quality of life of patients with endometrial cancer who are disease-free following external irradiation. Acta Oncol. 2001;40:816–824.
- Klopp AH, Yeung AR, Deshmukh S, et al. A phase III randomized trial comparing patient-reported toxicity and quality of life (QOL) during pelvic intensity modulated radiation therapy as compared to conventional radiation therapy. Int J Radiat Oncol. 2016;96:S3.
- de Boer SM, Powell ME, Mileshkin L, et al. Toxicity and quality of life after adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17:1114–1126.
- Chen CC, Wang L, Lu CH, et al. Comparison of clinical outcomes and toxicity in endometrial cancer patients treated with adjuvant intensity-modulated radiation therapy or conventional radiotherapy. J Formos Med Assoc. 2014;113:949–955.
- Mundt AJ, Roeske JC, Lujan AE, et al. Initial clinical experience with intensity-modulated whole-pelvis radiation therapy in women with gynecologic malignancies. Gynecol Oncol. 2001;82:456–463.
- Mundt AJ, Mell LK, Roeske JC. Preliminary analysis of chronic gastrointestinal toxicity in gynecology patients treated with intensity-modulated whole pelvic radiation therapy. Int J Radiat Oncol Biol Phys. 2003;56:1354–1360.
- Cho LP, Cheng Z, McNutt TR, et al. Comparison of prospectively-collected genitourinary and gastrointestinal toxicities between post-operative intensity-modulated radiation therapy and non-intensity-modulated radiation therapy patients. Int J Radiat Oncol. 2017;99:E286–E287.
- Foerster R, Schnetzke L, Bruckner T, et al. Prognostic factors for long-term quality of life after adjuvant radiotherapy in women with endometrial cancer. Strahlenther Onkol. 2016;192:895–904.
- Rovirosa Á, Cortés·KS, Ascaso·C, et al. Are endometrial cancer radiotherapy results age related? Clin Transl Oncol. 2018;20:1416–1421.
- Soisson S, Ganz PA, Gaffney D, et al. Long-term, adverse genitourinary outcomes among endometrial cancer survivors in a large, population-based cohort study. Gynecol Oncol. 2018;148:499–506.
- Heron DE, Gerszten K, Selvaraj RN, et al. Conventional 3D conformal versus intensity-modulated radiotherapy for the adjuvant treatment of gynecologic malignancies: a comparative dosimetric study of dose-volume histograms. Gynecol Oncol. 2003;91:39–45.
- Canlorbe G, Bendifallah S, Laas E, et al. Tumor size, an additional prognostic factor to include in low-risk endometrial cancer: results of a french multicenter study. Ann Surg Oncol. 2016;23:171–177.
- Bendifallah S, Canlorbe G, Collinet P, et al. Just how accurate are the major risk stratification systems for early-stage endometrial cancer? Br J Cancer. 2015;112:793–801.