Abstract
Background: Early mortality is a major deterrent to oncologic management, often preventing delivery of therapy or leading to administration of treatment that offers limited benefit from aggressive interventions. Due to more recent progress in therapeutic options for stage IV non-small cell lung cancer (NSCLC) patients, identifying those at high risk of early mortality (within 30 days) could have implications for treatment selection. Because early mortality following diagnosis of metastatic non-small cell lung cancer (NSCLC) is not well-characterized, this investigation evaluated national trends and predictors thereof.
Material and methods: The National Cancer Database was queried for cases of pathologically confirmed metastatic NSCLC with complete vital status and clinical information, diagnosed between 2006 and 2014. Multivariable logistic regression ascertained factors associated with 30-day mortality.
Results: Of 346,681 patients, 45,861 (13%) experienced early mortality over the past decade, which remained relatively constant over time. Predictors of early mortality included advancing age (>65 years), male gender, Caucasian race, non-private insurance, lower income, greater comorbidities, residence in metropolitan and/or lesser-educated areas, treatment at community centers, patients with no prior history of cancer and regional differences (p < .01 for all). Early mortality was highest in patients older than 80 years with multiple comorbidities (29%). The majority of patients (71%) who died within 30 days did not receive any therapy.
Conclusions: A fair proportion of NSCLC patients experience early mortality, which has not decreased over time. The majority of patients with early mortality do not receive treatment. Prognostic factors for early mortality should be considered during initial evaluation and subsequent follow-up of these patients. Doing so may impact systemic treatment selection by medical oncologists, management of (oligo)metastatic disease by radiation and surgical oncologists and cost-effective administration of these therapies in the stage IV NSCLC population.
Introduction
Non-small cell lung cancer (NSCLC) is associated with a relatively poor prognosis, not only owing to the high cancer-related mortality, but also from smoking- and age-related comorbidities [Citation1–3]. Approximately 60% of all NSCLC patients present with metastatic disease at diagnosis [Citation4]. Traditionally, systemic chemotherapy was the treatment of choice for these patients. In the more recent era however, molecular testing has become standard in patients with advanced or metastatic disease [Citation5]. The addition of newer targeted agents for various mutations including epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS1, and BRAF has led to a modest improvement in survival for stage IV NSCLC patients [Citation6–8]. Further, PD-L1 testing to evaluate the role of immunotherapy has also become standard in these patients [Citation9]. Last, recent data suggest further improvement in progression-free survival (PFS) and overall survival (OS) with the addition of stereotactic body radiation therapy (SBRT) in stage IV NSCLC patients with oligometastatic disease [Citation10–12].
The recent paradigm shift has led to more aggressive treatment than the historical approach based on palliative chemotherapy. In light of this progress in therapeutic options for stage IV patients, identifying those at high risk of early mortality could have implications for treatment selection, as a minority of patients may not necessarily benefit from aggressive therapy. Better patient risk stratification is needed to differentiate those at high risk for early mortality compared to those who are not, as these individuals will benefit more from aggressive treatment, given 79–89% of NSCLC subjects who die within a year of diagnosis do so from disease (rather than comorbidities) [Citation1,Citation3].
Currently, there are very limited data predicting early mortality in patients diagnosed with stage IV NSCLC. With recent randomized data described above suggesting additional local modalities with systemic therapy improve OS, the expectation is more aggressive therapies will be offered to this cohort of patients. To help guide clinicians in properly selecting those who would benefit more from aggressive therapy versus best supportive care or systemic treatment alone, this study evaluates trends in early (30-day) mortality in the past decade, as well as predictors thereof, for patients diagnosed with upfront stage IV NSCLC in the USA.
Material and methods
The National Cancer Database (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society that consists of information regarding tumor characteristics, patient demographics and patient survival for approximately 70% of the U.S. population [Citation13]. The data used in this study were derived from a de-identified NCDB file. The American College of Surgeons and the CoC have not verified and are neither responsible for the analytic or statistical methodology employed nor the conclusions drawn from these data. As all patient information in the NCDB database is de-identified, this study was exempt from Institutional Review Board evaluation.
The NCDB Participant User File corresponding to non-small cell lung cancer (2004–2014) was utilized for this investigation. Patients were excluded if there was no information on follow-up and/or vital status, or if they were diagnosed prior to 2006 as the purpose of the study was to evaluate early mortality trends in the last 10 years. All non-metastatic patients or those with unknown clinical stage were also excluded leaving only those with clinical stage IV disease. Because the goal of this work was to evaluate factors associated with early mortality, defined as death within 30 days of NSCLC diagnosis, patients with missing clinical characteristics (as coded by the NCDB) were also removed. Systemic therapy, either chemotherapy or immunotherapy, was reported based on the following coding: https://seer.cancer.gov/tools/seerrx/. Receipt of radiation treatment was also included. Surgery was defined as wedge resection or greater: http://ncdbpuf.facs.org/content/surgery-primary-site-codes. Institution type was classified as community cancer program (more than 100 but fewer than 500 newly diagnosed cases per year), comprehensive community cancer program (500 or more newly diagnosed cancer cases each year), academic/research program including National Cancer Institute (NCI)-designated comprehensive cancer centers (participates in postgraduate medical education and/or receives NCI level funding, and treats more than 500 newly diagnosed cases each year) and integrated network cancer program (organization that owns, operates, leases or is part of a joint venture with multiple facilities) (https://www.facs.org/quality-programs/cancer/coc/apply/categories). Patient comorbidities were categorized as 0, 1, 2 or ≥3 according to Charlson–Deyo (CD) comorbidity scores [Citation14].
In accordance with the variables in NCDB files, information collected on each patient broadly included demographic, clinical and treatment data. Sensitivity analyses were performed for patients who were excluded and no statistically significant differences were seen in the group excluded compared to those included for patient characteristics. Statistical analysis was performed using SPSS V24.0 (SPSS Inc, Chicago, IL, USA). Tests were two-sided, with a threshold of p < .05 for statistical significance. First, clinical characteristics of the overall cohort were tabulated; inter-group comparisons were made with the chi-squared test. Next, multivariable logistic regression analysis was performed to ascertain factors independently associated with experiencing 30-day mortality in all patients. Variables included in the multivariable model were selected a priori and based on clinical significance. The Hosmer–Lemeshow test was used to check for the goodness-of-fit of the regression models.
Subgroup analyses by age and comorbidity score were performed and percentage of early mortality for each subgroup out of the total number of patients in that subgroup were calculated. A separate subgroup analysis was performed for patients who underwent treatment.
Results
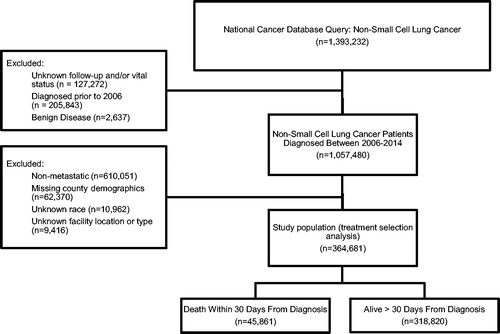
A patient selection diagram is illustrated in . A total of 364,681 patients were included, of whom 45,861 (13%) subjects experienced early mortality. The majority of patients who died within 30 days of diagnosis were 65 years and older (72%) with multiple comorbidities (52%) (). Treatment modalities for those dying within 30 days of diagnosis were the following: no treatment (71%), radiation alone (19%) and systemic treatment alone (6%). Treatment modalities for those alive longer than 30 days were the following: systemic treatment and radiation (31%), systemic treatment alone (29%), no treatment (20%) and radiation alone (18%) (). The proportion of stage IV patients experiencing 30-day mortality has not appreciably changed with time over the study period, ranging between 12 and 13%.
Table 1. Patient and treatment characteristics.
Multivariable logistic regression analysis was performed to evaluate independent predictors associated with early mortality (). Predictors associated with higher risk for early mortality included advanced age (odds ratio [OR] 1.55; 95% confidence interval [CI] 1.51–1.60), non-private insurance including government-type (OR 1.28; 95% CI 1.24–1.32) and uninsured (OR 1.25; 95% CI 1.20–1.31), higher comorbidity scores (ORs 1.57–2.64) and treatment in the Midwest (OR 1.04; 95% CI 1.01–1.07), South (OR 1.09; 95% CI 1.05–1.12) or Eastern states (OR 1.07; 95% CI 1.03–1.11) when compared to the West (reference). Factors associated with a lower risk for early mortality included female gender (OR 0.83; 95% CI 0.82–0.85), African-American (0.85; 95% CI 0.83–0.88) or Other race (OR 0.71; 95% CI 0.66–0.75) when compared to White race (reference), residence in urban (OR 0.91; 95% CI 0.89–0.94) or rural (OR 0.91; 95% CI 0.85–0.97) residence when compared to metropolitan (reference), patients from higher income counties (OR 0.89–0.93), those from counties with higher rates of high-school graduates (OR 0.94; 95% CI 0.90–0.98) and treatment at comprehensive community programs (OR 0.96; 95% CI 0.93–0.99), academic/research centers (OR 0.77; 95% CI 0.75–0.80) or integrated cancer networks (OR 0.91; 95% CI 0.88–0.95) when compared to community cancer programs (reference). In addition to these differences, patients with a prior history of cancer were less likely to die within 30 days of diagnosis (OR 0.83; 95% CI 0.81–0.85).
Table 2. Logistic regression analysis of predictors for 30 day mortality from time of diagnosis.
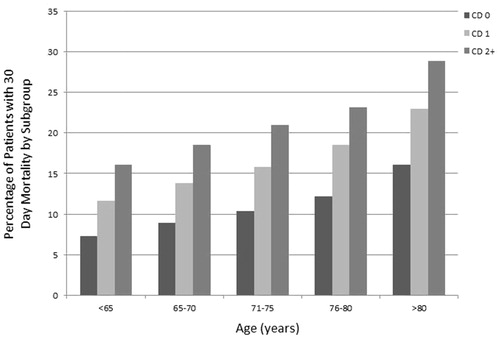
Subgroup analyses of 30-day mortality were calculated by age and comorbidity score. The cohort with the lowest rate of early mortality were patients younger than 65 years old with a comorbidity score of 0 (6855 out of 94,119 patients; 7.3%). Those patients older than 80 years with a comorbidity score ≥2 (2076 out of 7183 patients; 28.9%) had the highest rates of early mortality ().
Figure 2. Subgroup analyses by age and comorbidity score with representative percentages of early (30-day) mortality for each subgroup out of the total number of patients in that subgroup. CD (Charlson–Deyo) comorbidity score.
On subgroup analysis for patients who received treatment, predictors for early mortality included older age (OR 1.15; 95% CI 1.05–1.27), higher comorbidity burden (ORs 1.65–2.53), treatment at a comprehensive community program (OR 1.14; 95% CI 1.03–1.26), treatment in the Midwest (OR 1.19; 95% CI 1.08–1.32), South (OR 1.22; 95% CI 1.11–1.36) or Eastern states (OR 1.17; 95% CI 1.04–1.33) when compared to the West (reference) and adenocarcinoma histology (OR 1.20; 95% CI 1.10–1.31). Factors associated with a lower risk for early mortality included female gender (OR 0.77; 95% CI 0.72–0.82), African-American (0.73; 95% CI 0.64–0.82) or Other race (OR 0.73; 95% CI 0.59–0.90) when compared to White race (reference), treatment at academic/research centers (OR 0.78; 95% CI 0.69–0.88). Patients again with a prior history of cancer were less likely to die within 30 days of diagnosis (OR 0.83; 95% CI 0.76–0.90) (Supplementary Table S1).
Discussion
The increasing implementation of multiple therapy options including targeted agents, immunotherapy and local therapy (SBRT, surgery) for stage IV NSCLC necessitates dedicated investigations evaluating trends in early mortality and predictors thereof. Using a large, contemporary national database of NSCLC patients, we observed that a meaningful proportion (13%) of metastatic NSCLC patients diagnosed in the past decade experienced early mortality, which has unfortunately not changed appreciably over time. Most of these patients (71%) did not receive any treatment. There were several prognostic factors for 30-day mortality, which should be considered during initial clinical evaluation and subsequent follow-up of these patients. The strongest predictors appeared to be age and comorbidity score. For patients older than 80 years, with a Charlson comorbidity score of 2 or higher, 29% had early mortality, compared to the younger, healthier newly diagnosed patient (7%). These results have implications on the implementation of systemic treatment options early at diagnosis, treatment of (oligo)metastatic disease with radiation therapy and cost-effectiveness.
The paradigm for stage IV NSCLC has rapidly changed over the past few years with the advent of immunotherapy [Citation9] and molecularly targeted agents [Citation15]. Additionally, within this past year, multiple randomized trials suggest improved PFS [Citation11] and OS with the addition of SBRT in patients with oligometastatic disease [Citation12,Citation16]. However, application of newer targeted therapies including immunotherapy with modalities including SBRT to clinical practice is notably problematic because stage IV NSCLC (even using per-protocol inclusion criteria) is a highly heterogeneous disease. In other words, not all patients eligible for inclusion in those randomized trials achieve a benefit to the same degree, and careful patient selection – going beyond disease characteristics alone – remains paramount to ensuring appropriate delivery of aggressive oncologic care. For instance, despite the inclusion criteria of KEYNOTE-024, immunotherapy is unlikely to benefit stage IV patients with advanced age, poor performance status and significant comorbidities to a clinically meaningful extent. Similarly, oligometastatic patients should represent a distinct entity with distinct prognostic factors worth consideration when delivering more aggressive oncologic therapy [Citation17,Citation18]. To this extent, given the relatively high rates of early mortality in stage IV NSCLC, the factors predicting for early mortality in stage IV cases such as older age and presence of multiple comorbidities should assist in ‘painting a clinical picture’ of patients who are at high risk of dying shortly after diagnosis and thus may not benefit from aggressive therapies. With nearly 30% of patients older than 80 years with multiple comorbidities dying within 30 days of their diagnosis of metastatic NSCLC, better attention to this population is needed. Attempts are being made to better risk-stratify these patients with geriatric assessment tools to potentially offer more tailored approaches to treatment [Citation19, Citation20]. Older adults pose a challenge for oncologists who need to weight the expected oncologic benefits from treatment and the risks from treatment-related side effects.
These findings also impact cost-effectiveness profiles for interventions for stage IV NSCLC. Cost-effectiveness is highly dependent on whether a patient will live long enough to benefit from expensive oncologic interventions. This means that careful patient selection is the most important determinant of economic feasibility [Citation21, Citation22]. To this extent, the results of this study may better inform judicious, cost-effective utilization of aggressive therapies in both the stage IV and overall NSCLC populations by identifying factors portending a high risk of early mortality. The prognostic factors for early mortality identified herein are consistent with those corresponding to general mortality [Citation23, Citation24], but a large proportion of patients herein would not be eligible for randomized trials of immunotherapy or SABR given the distribution of age and comorbidities of a ‘real-world’ cohort such as in this investigation. To this extent, clinicians must bear in mind that the results of a positively interpreted randomized trial may not apply to ‘real-world’ patients equivalently, given that trial patients tend to be younger, healthier, and more ‘oncologically favorable’ [Citation25]. In this study, the majority of patients who died within 30 days did not receive any form of treatment, suggesting that in the past decade, physicians are properly selecting patients for treatment as older individuals with multiple comorbidities may have more risk than benefit with chemotherapy. In the era of immunotherapy however which is felt to be better tolerated in older patients, this pattern may change, though comorbidities will continue to be an issue [Citation26]. Nevertheless, patient factors especially age and comorbidities must be factored into the treatment decision algorithm which this study suggests.
Limitations of the NCDB must be acknowledged. In addition to retrospective selection biases, the NCDB does not carry information on causes of death, response to therapies, performance status, smoking status, weight loss and other known prognostic factors. The NCDB also does not contain information on the number or volume of metastases. Further, there is no information on the reason for treatment failure or cause of death. The database also lacks data on type of systemic therapy received. Last, although the NCDB includes a large proportion of the U.S. population, only CoC-accredited facilities contribute data; as such, these findings may not necessarily be representative of the entire U.S. population.
Conclusions
Using a large, contemporary national database of nearly 1 million NSCLC patients diagnosed in the past decade in the USA, we observed that a fair proportion (13%) of these patients experience early mortality, which has unfortunately not changed appreciably over time. There were several prognostic factors for 30-day mortality in the overall NSCLC population including older age and presence of medical comorbidities, which should be considered during initial clinical evaluation and subsequent follow-up of these patients. The majority of these patients did not receive any therapy suggesting overall that physicians are properly selecting patients who would or would not benefit from treatment. These results have implications on the early intervention for stage IV NSCLC patients, systemic treatment selection by medical oncologists, management of (oligo)metastatic disease by radiation and surgical oncologist and cost-effectiveness.
Disclaimers
None. A portion of this work was presented at the 2018 meeting of the American Society for Radiation Oncology.
Supplemental Material
Download MS Word (20.1 KB)Disclosure statement
All authors declare that conflicts of interest do not exist.
Additional information
Funding
References
- Janssen-Heijnen ML, Smulders S, Lemmens VE, et al. Effect of comorbidity on the treatment and prognosis of elderly patients with non-small cell lung cancer. Thorax. 2004;59:602–607.
- Jorgensen TL, Hallas J, Friis S, et al. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer. 2012;106:1353–1360.
- Janssen-Heijnen ML, van Erning FN, De Ruysscher DK, et al. Variation in causes of death in patients with non-small cell lung cancer according to stage and time since diagnosis. Ann Oncol. 2015;26:902–907.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
- National Comprehensive Cancer Network (NCCN) Lung Guidelines [Internet]. [cited 2018 Dec 19]. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx#nscl
- Besse B, Adjei A, Baas P, et al. 2nd ESMO Consensus Conference on Lung Cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol. 2014;25:1475–1484.
- Sandler AB, Johnson DH, Herbst RS. Anti-vascular endothelial growth factor monoclonals in non-small cell lung cancer. Clin Cancer Res. 2004;10:4258s–4262s.
- Giaccone G. Epidermal growth factor receptor inhibitors in the treatment of non-small-cell lung cancer. J Clin Oncol. 2005;23:3235–3242.
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833.
- Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy (LCT) improves overall survival (OS) compared to maintenance therapy/observation in oligometastatic non-small cell lung cancer (NSCLC): final results of a multicenter, randomized, controlled phase 2 trial. Int J Radiat Oncol Biol Phys. 2018;102:1604.
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018;4:e173501.
- Palma DA, Olson RA, Harrow S, et al. Stereotactic ablative radiation therapy for the comprehensive treatment of oligometastatic ss (SABR-COMET): results of a randomized trial. Int J Radiat Oncol Biol Phys. 2018;102:S3–S4.
- Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619.
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125.
- Gomez DR, Blumenschein GR, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17:1672–1682.
- Mendez JA, Benjamín D, Lorente D, et al. Prognostic factors in oligometastatic non-small-cell lung cancer (OM-NSCLC) patients treated with ablative therapy. JCO. 2018;36:e21089.
- Li S, Zhu R, Li D, et al. Prognostic factors of oligometastatic non-small cell lung cancer: a meta-analysis. J Thorac Dis. 2018;10:3701–3713.
- NCCN Guidelines. Older adult oncology. Version 2 [Internet]. [cited 2018 Dec 26]. Available from: https://www.nccn.org/
- Hamaker ME, Jonker JM, de Rooij SE, et al. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol. 2012;13:e437–e444.
- Verma V. Economic sustainability of immune-checkpoint inhibitors: the looming threat. Nat Rev Clin Oncol. 2018;15:721–722.
- Verma V, Shah C, Rwigema JC, et al. Cost-comparativeness of proton versus photon therapy. Chin Clin Oncol. 2016;5:56.
- Ma M, Shen J, Jiang L, et al. Prognostic factors in patients with stage IV non-small cell lung cancer. Chinese German J Clin Oncol. 2006;5:319–323.
- Skaug K, Eide GE, Gulsvik A. Predictors of long-term survival of lung cancer patients in a Norwegian community. Clin Respir J. 2011;5:50–58.
- Unger JM, Cook E, Tai E, et al. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book. 2016;35:185–198.
- Helissey C, Vicier C, Champiat S. The development of immunotherapy in older adults: new treatments, new toxicities? J Geriatr Oncol. 2016;7:325–333.