Abstract
Background: The treatment options for patients with therapy refractory metastatic colorectal cancer (mCRC) are sparse. TAS-102 (FTD/TPI) is a new oral anti-tumour agent composed of a nucleoside analogue, trifluridine, and a thymidine phosphorylase inhibitor, tipiracil, indicated for patients with mCRC who are refractory to standard therapies. This study summarizes published and unpublished experience with FTD/TPI in clinical practice settings.
Patients and methods: The Medline/PubMed, Embase and Cochrane Library databases were searched to identify observational studies on FTD/TPI monotherapy for mCRC. Papers describing use of FTD/TPI monotherapy outside clinical trials in series of patients evaluable for effectiveness were eligible. The outcomes of interest were median progression free survival (mPFS), median overall survival (mOS) as well as mean PFS time restricted to six months (PFS6m) and mean OS time restricted to one year (OS1y). Results of the pooled analyses of observational studies were compared to the results of the Japanese phase II trial and the two phase III trials, RECOURSE and TERRA.
Results: Seven published and two unpublished studies with 1008 patients from 64 centres were included for analysis. The pooled mPFS was 2.2 months (95% CI 2.1 to 2.3 months), and the pooled mOS was 6.6 months (95% CI 6.1 to 7.1 months). PFS6m was 2.9 months (95% CI 2.6 to 3.1 months) and OS1y was 6.8 (95% CI 6.0 to 7.5) months. While these results all reflect RECOURSE, the pooled mOS is lower than in the phase II trial and the OS1y is inferior to both the phase II trial and TERRA.
Conclusion: This systematic review and a meta-analysis indicates that in real life settings, the survival benefit of FTD/TPI monotherapy in patients with therapy refractory mCRC reflects the outcomes in RECOURSE but is inferior to outcomes in the two Asian efficacy trials.
TAS 102 (Lonsurf) is an oral fixed dose combination of trifluridine (FTD) and tipiracil (TPI) indicated as salvage-line treatment in patients with therapy refractory metastatic colorectal cancer (mCRC). A Japanese phase II trial and two phase III trials, RECOURSE and TERRA, demonstrated that FTD/TPI prolonged overall survival.
What is already known
This systematic review and meta-analysis of real life data from 64 sites indicates that the effectiveness in daily clinical practice settings of FTD/TPI monotherapy in late stage mCRC reflects the outcomes in RECOURCE but is inferior to the outcomes in the Japanese phase II trial and TERRA.
What this study adds
Introduction
TAS-102 (FTD/TPI) is an oral combination of the antineoplastic nucleoside analogue trifluridine (FTD) and the thymidine phosphorylator inhibitor tipiracil (TPI). The primary cytotoxic mechanism is DNA dysfunction through incorporation of tri-phosphorylated FTD into DNA by thymidine kinase. This mechanism of action is distinct from the inhibition of thymidylate synthase caused by 5-FU and other fluoropyrimidines [Citation1]. Reducing degradation of FTD, TPI improves the bioavailability and ensures sufficient blood concentrations of FTD. FTD/TPI is indicated for patients with metastatic colorectal cancer (mCRC) who have been treated with fluoropyrimidine-, oxaliplatin-, or irinotecan-based chemotherapies and antibodies targeting vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR).
FTD/TPI was approved for refractory mCRC in Japan (March 2014) [Citation2] and subsequently, the RECOURSE trial led to approval in the United States (September 2015) and Europe (April 2016) [Citation3]. The efficacy and safety of FTD/TPI monotherapy in adults with refractory mCRC was first demonstrated in a Japanese phase II trial by Yoshino et al. [Citation4] and later in the pivotal phase III RECOURSE trial [Citation5]. The latter study showed that compared with placebo, FTD/TPI increased median overall survival (mOS) from 5.3 to 7.1 months (p < .001) and median progression free survival (mPFS) from 1.7 to 2.0 months (p < .001). Recently, a second phase III trial in an entirely Asian population, TERRA, confirmed these results [Citation6]. Patients enrolled in these efficacy studies were adults with mCRC who had had two or more regimens of standard chemotherapy, and in whom fluoropyrimidine, irinotecan, and oxaliplatin did not work or were unsuitable. In RECOURSE, but not in Yoshino et al. or TERRA, patients must also have had bevacizumab before enrolling in the study while patients with KRAS wild-type tumours must have had an anti-EGFR antibody.
The primary goal of the efficacy trials was to establish efficacy and safety of FTD/TPI in carefully selected groups of patients. In clinical use, however, new drugs are typically used in a less standardized manner to a more heterogeneous patient population [Citation7]. This means that even though the evidence from the clinical trials favours FTD/TPI, it may not inform routine clinical practice if the efficacy demonstrated in clinical trials differs from the effectiveness that can be achieved in real life settings [Citation8]. To determine if the efficacy of FTD/TPI can be replicated in real life circumstances, we need effectiveness studies that provide results that are more applicable to clinical practice [Citation7]. Since approval of FTD/TPI, several observational studies have been published that describe the experience with FTD/TPI outside the context of a clinical trial and recently, Yoshino et al. reviewed real life safety of FTD/TPI [Citation2]. The aim of the present study was to conduct a systematic review of observational studies on the effectiveness of FTD/TPI in clinical practice settings. Second, we conducted a meta-analysis of these trials and compared the effectiveness of FTD/TPI to the efficacy reported in Yoshino et al. [Citation4] and the two phase III trials, RECOURSE [Citation5] and TERRA [Citation6].
Material and methods
Using the terms trifluridine and tipiracil, TAS-102, and Lonsurf, we searched Medline/PubMed, Embase and Cochrane Library databases up to 19 June 2018 to identify published articles on FTD/TPI for mCRC. Observational studies in English, German, or Danish describing clinical experience with FTD/TPI monotherapy in series of patients evaluable for effectiveness were included. We excluded papers reporting phases I, II, or III trials, case reports and series with less than 10 patients, and publications in other languages or available only in abstract form. To locate additional relevant publications, we searched the references of the retrieved articles manually. Furthermore, we contacted members of the five Danish regional drug and therapeutic committees (D&TC) and authors of published conference abstracts by email to identify unpublished data series.
The risk of bias within each of the included studies was assessed using the JBI critical appraisal checklist for cohort studies [Citation9]. Publication bias was assessed from visual inspection of funnel plots for mPFS and mOS.
From each of the included studies two authors (SEA and IBA) extracted following demographic data: age, gender, Eastern Cooperative Oncology Group performance status (ECOG PS), location of the primary tumor, KRAS mutational status, time from diagnosis of metastatic disease to FTD/TPI treatment, and number and type of previous lines of therapy for mCRC. The extracted effectiveness outcomes comprised mPFS and mOS. Supplementary Information on the actual response evaluation frequency and the ethnicity of the study patients was requested from the corresponding authors of all the included studies. One author extracted data and a second author reviewed the extracted data to ensure accuracy. Discrepancies were discussed and resolved cooperatively. For the study characteristics, we computed descriptive statistics and used odds ratios with 95% confidence intervals (CI) to compare with the FTD/TPI arms of the three efficacy trials. Since some of the included studies did not provide complete population data, we present the number of studies and total number of patients from which each outcome of interest was derived.
All identified observational studies were included in the primary analyses and the Asian series were included in a secondary meta-analysis. We used Cochran’s Q and I2-test to evaluate heterogeneity amongst studies and when low, we used a fixed effect model to calculate the pooled summary statistics. Otherwise, we used a random effects model. If not explicitly provided, standard errors were calculated from the confidence intervals. One study [Citation10] failed to provide standard errors or confidence intervals and the standard errors were imputed from variance observed in the other included studies. All calculations were performed in Excel (Microsoft Corporation, Redmond, WA, USA) and based on a method described by Neyeloff et al. [Citation11].
When the included papers reported Kaplan–Meier curves, we used the Automeris software (apps.automeris.io/wpd) to scan and reconstruct the PFS and OS survival curves. From unpublished series, we extracted individual patient data and used software R version 3.5.0 (The R Foundation for Statistical computing) to generate survival curves using Kaplan–Meier estimates (Supplementary Appendix 1).
Subsequently, we integrated the Kaplan–Meier curves using the trapezoidal method. From these areas we calculated the restricted mean survival times (RMST) that is the area under the survival curve from t = 0 limited to some time horizon [Citation12]. The length of follow-up in the included studies varied. Thus, to be able to include data from all studies with Kaplan–Meier curves, we chose a six month time horizon for the mean PFS time (PFS6m) and a 12 month time horizon for the mean OS time (OS1y). Subsequently, we calculated the weighted averages of PFS6m and OS1y with 95% CI [Citation13] in Excel using the square root of the size of the study as weight. Secondly, we calculated PFS6m and OS1y of the Asian series only (five published and one unpublished). PFS6m and OS1y express the average number of months future patients are expected to live without progression and the average number of months they are expected to survive out of the first six and twelve months of follow-up, respectively.
Results
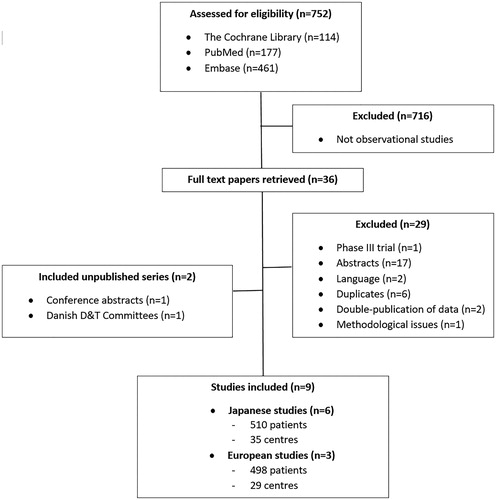
Initially, 752 publications were retrieved and assessed for eligibility by title and abstract, 716 of which were not observational studies and, therefore, not included (). Additionally, 20 publications were excluded because either they described phases I, II, or III trials, were conference abstracts, or they were not written in English, German, or Danish. Moreover, eight studies were excluded that were duplicates or described data that had been published twice. Finally, one study was excluded due to methodological flaws.
In total, seven published and two unpublished studies with 1008 patients from 64 Japanese and European centres were included (). The studies were published between 2016 and 2018 (). The unpublished series were Japanese and Danish (Supplementary Appendix 1). The former was identified from a conference abstract [Citation14] and comprised individual data on 17 patients from Izumi City General Hospital who had received FTD/TPI monotherapy for chemo-refractory mCRC from July 2014 to June 2018. The latter series had been collected by the Danish D&TCs and comprised data on all non-trial patients (n = 21 from four sites) who had received FTD/TPI for chemo-refractory mCRC from January 2017 to June 2018 outside clinical trials. The D&TCs had collected these anonymised, retrospective data from the attending oncologists to monitor the treatment quality and effectiveness in real life settings because the Danish Council for Coordinated Use of Hospital Drugs in June 2016 rejected FTD/TPI as standard therapy but allowed limited use based on individual clinical assessment.
Table 1. The included studies.
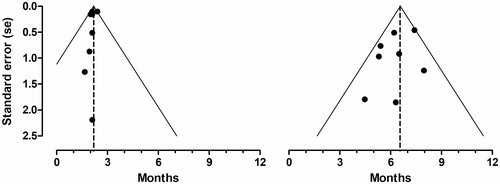
Visual inspection of the funnel plots for mPFS and mOS () revealed no asymmetry suggesting that publication bias was not an influential factor. The methodological study quality was generally good (Supplementary Appendix 2). Four studies, however, reported no details on actual dose intensity [Citation15–18], two studies reported no data on the assessment of tumor progression [Citation16,Citation17], and two studies did not address confounding factors [Citation19,Citation20].
Figure 2. Funnel plots. Funnel plots with pseudo 95% confidence limits for median progression free survival (left) and median overall survival (right). The dotted lines illustrate the pooled estimates.
Depending on the patient characteristics, 5–9 of the studies provided adequate information (). Most of the patients (93%) included in the observational studies had good performance status (ECOG PS 0 or 1), the majority were men, and in 60% colon was the primary site of tumor. About one third of the patients had received one or two previous lines of therapy, one third had received three previous lines, and a third had received four or more previous lines. More than 90% of the patients for whom data were available had been treated with 5-flouropyrimidines, irinotecan, and oxaliplatin. The majority had also received bevacizumab or ramucirumab while 40% had received cetuximab or panitumumab. Furthermore, nearly one third of the patients had received regorafenib. Compared to the RECOURSE population, the patients in the observational studies had poorer performance status, more had KRAS mutations, and more had had metastatic disease for less than 18 months when receiving FTD/TPI. However, the included patients had received fewer previous lines of therapy, fewer patients had been exposed to anti-EGFR monoclonal antibodies, and more had received regorafenib. Compared to the Japanese phase II population, the patients in the observational studies had poorer performance status, more had KRAS mutations, and fewer had received anti-EGFR antibodies. Compared to the TERRA population, the patients in the observational studies had higher performance status, more had KRAS mutations, and fewer had had metastatic disease for less than 18 months. They had also received fewer previous lines of therapy but more had received anti-EGFR monoclonal antibodies.
All nine included studies reported mPFS. Between study heterogeneity was very low (I2 = .00, Cochranes Q = 7.83, p = .45) and, therefore, a fixed effect model was applied. The pooled mPFS was 2.2 months (95% CI 2.1 to 2.3 months). Data on mOS was also available from all included studies. Heterogeneity was low to moderate (I2 = 28.6, Cochranes Q = 11.2, p = .19) and a fixed effect model was applied. The pooled mOS was 6.6 months (95% CI 6.1 to 7.1 months). A random effects model produced a similar estimate of mOS (6.5 months (95% CI 5.8 to 7.2 months)). While the pooled mPFS is in line with the outcomes in the FTD/TPI-arms of the three efficacy trials, the pooled mOS is inferior to the mOS in Yoshino et al. (). shows the forest plots.
Figure 3. Forest plots. Pooled analyses of median progression free survival (top) and median overall survival (bottom). A black box that also gives a representation of the size of the study represents point estimates of the individual studies with 95% confidence intervals. The white summary diamond shows the pooled estimate from a fixed effect model. The grey dots at the bottom represents point estimates from the trifluridine/tipiracil-arms of the three efficacy studies with 95% confidence intervals.
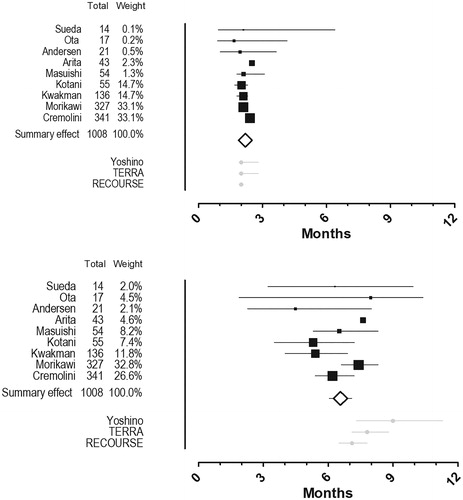
Table 3. Median progression free survival (mPFS), median overall survival (mOS), and restricted mean survival times in the phases II and III trial and the current analysis of observational studies.
Including only the six Asian series with 510 patients, the pooled mPFS was 2.1 months (95% CI 1.9 to 2.2 months) and the pooled mOS was 7.0 (95% CI 6.4 to 7.7 months). Both outcomes reflect the outcomes in all of the three efficacy studies ().
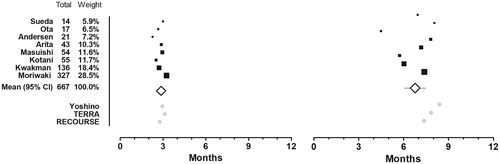
Six published [Citation10,Citation15–17,Citation19,Citation20] and two unpublished studies (Supplementary Appendix 1) with a total of 667 patients provided Kaplan–Meier curves and informed the calculation of average restricted mean survival times (). The PFS6m was 2.9 months (95% CI 2.6 to 3.1 months) and the OS1y was 6.8 months (95% CI 6.0 to 7.5 months). Both PFS6m and OS1y reflect the FTD/TPI-arm of REOURSE but OS1y is inferior to the outcomes from both of the Asian efficacy studies (). Including only the six Asian series, the PFS6m was 3.0 months (95% CI 2.7 to 3.2 months) and the OS1y was 7.2 months (95% CI 6.6 to 7.8 months). Although, the OS1y in the Asian studies better reflects both phase III trials, it is still inferior to the outcome in Yoshino et al. (.)
Figure 4. Mean restricted survival time. Mean progression free survival time restricted at 6 months (left) and overall survival time restricted at 12 months (right). A black box that also gives a representation of the size of the study represents point estimates of the individual studies. The white summary diamond shows the weighted mean with 95% confidence interval. The grey dots at the bottom represents point estimates from the trifluridine/tipiracil-arms of the three efficacy studies.
Discussion
Worldwide, colorectal cancer is among the most common cancers [Citation21] and most common causes of cancer death [Citation22]. Almost 55% of the cases occur in the more developed countries [Citation22]. The treatment options include fluoropyrimidines, oxaliplatin, and irinotecan, as well as monoclonal antibodies targeting the angiogenesis (bevacizumab and ramucirumab) or in RAS wild-type tumours the EGF-receptor (cetuximab and panitumumab). In patients not responding to these therapies, only two treatment options are currently available: regorafenib and FTD/TPI. Recently, the effectiveness and safety of regorafenib was reviewed [Citation23]. In the present study, we analyse real life experience with FTP/TPI from more than 1000 patients treated in 64 centres and report that FTD/TPI is associated with a mPFS of 2.2 months (95% CI 2.1 to 2.3 months) and a mOS of 6.6 months (95% CI 6.1 to 7.1 months). Since the included studies are uncontrolled, our data do not allow quantification of the actual survival benefit of FTD/TPI in real life settings. Still, the survival of the non-trial patients in our study resembles the mOS of 7.1 and 7.8 months reported in the FTD/TPI-arm of both RECOURSE and TERRA ().
In the two Asian efficacy studies [Citation4,Citation6], the survival benefit of FTD/TPI tended to be higher than in RECOURSE where only 33% of the patients were Asian [Citation5]. Since we did not have access to individual patient data, we could not explore the impact of ethnicity. In four of the included studies (), information about ethnicity was unavailable. Therefore, to explore geographic variation, we conducted a secondary meta-analysis of the six Asian studies that revealed a higher mOS than in the primary meta-analysis. Despite partly overlapping confidence intervals, the mOS of 7.0 (95% CI 6.4 to 7.7) months in the subgroup of Asian studies does not fully compare to the mOS of 9.0 (95% CI 7.3 to 11.3) months reported by Yoshino et al. (). The aetiology of these disparities is multifactorial. Beyond ethnicity, it includes factors such as tumour characteristics, co-morbidity, lifestyle, and socioeconomic status [Citation24] none of which could be included in the present meta-analysis.
Although, the correlation between PFS and OS is poor [Citation25], PFS is a commonly used endpoint for third line trials in mCRC. Assessment of disease progression is typically based on radiologic testing. As the true progression time lies somewhere in the time interval between two assessments, the PFS is overestimated if the detection date is used as the date of progression [Citation26]. Thus, because the surveillance intervals may drive the PFS, comparison of median PFS across studies may be less meaningful if the scanning intervals are heterogeneous as could be the case in the present study where the exact scanning frequency was unknown in three of the included studies. This means that the reported PFS is probably biased and the pooled PFS should be interpreted cautiously. OS is more robust and here you can see more heterogeneity ( and ).
Median survival time is one of the most commonly reported endpoints for randomized controlled trials (RCT) in cancer. Nevertheless, median survival times are point estimates that summarize the survival curve by only one point corresponding to the survival of 50% of the patients. This measure does capture the entire temporal profile of the survival function and use of median survival times may result in underestimation or overestimation of the true treatment effect [Citation27]. Therefore, we determined the restricted mean survival time (RMST). RMST is an alternative summary measure that is useful in a meta-analysis of RCTs with time-to-event outcomes because it does not rely on the proportional hazards assumption [Citation28]. In oncology RCTs, HR and RMST-based measures agree regarding the direction of statistical effects but HR provides significantly larger treatment effect estimates than the ratio of RMST does [Citation29]. Since RMST complements the hazard ratio, it has been suggested that hazard rate (HR) and RMST should both be reported in trials with time-to-event outcomes [Citation29,Citation30]. This approach was used by Lacas et al. in a meta-analysis of radiotherapy in head and neck cancers [Citation31] and by Bullement et al. in a cost-effectiveness analysis of FTD/TPI [Citation32].
In uncontrolled studies with time-to-event outcomes, like those included in the present study, RMST is an easily interpretable absolute measure that express how long, on average, study patients lived before they experienced an outcome but RMST depends on the selection of truncation time. As we did not apply survival fitting to model outcomes beyond the observed data, we chose six months for PFS and one year for OS to be able to include data from all identified studies. Both seem clinical relevant in patients with mCRC. Our analyses showed that if treated with FTD/TPI in clinical settings, future patients with refractory-stage mCRC could expect to live 2.6 to 3.1 months before their disease progresses while their total life expectancy would be 6.0 to 7.5 months. Although, these results reflect the FTD/TPI-arm of RECOURSE, the OS1y is inferior to both Yoshino et al. and TERRA ().
Optimally designed for assessing the efficacy of a new drug, RCTs like RECOURSE and TERRA are conducted in artificial homogenous populations and the risk of bias is minimized. Moreover, RCTs are typically conducted in specialized settings in accordance with a detailed protocol [Citation33]. In the present study we included real-life, observational studies designed to evaluate effectiveness in less homogenous populations and these studies all suffer from the well-known shortcomings of retrospective and uncontrolled studies. The internal validity is lower than in a well-conducted RCT. On the other hand, methodologic issues like standardized treatment protocols, strict inclusion criteria such as age and performance status, free provision of medications, and compliance control also hamper the generalisability (external validity) of the results from RCTs [Citation34] which has often frustrated clinicians [Citation35]. This emphasises the need for studies that examine the effectiveness of new drugs like FTD/TPI in broader defined patient populations treated outside of clinical trials where prescribers meet fewer restrictions to modify doses, regimens, or co-therapy. The potential higher external validity of effectiveness studies, however, is obtained at the expense of the internal validity. Still, both efficacy- and effectiveness-studies are required: Efficacy studies show whether an intervention can work under ideal circumstances and effectiveness studies show if it also works in daily practice.
The term ‘real-life data’ covers a range of research methodologies [Citation8]. In the present study, we primarily included uncontrolled retrospective cohort studies and assessed as such, the study quality was fair. No funnel plot asymmetry as an indication of publication bias was detected. Compared to the efficacy trial participants, the population in the present study was less homogenous and differed in terms of performance status, frequency of KRAS mutations, time since diagnose of the first metastases, and previous treatment. Since only the two unpublished data series provided individual data, we could not explore the impact of these differences on the effectiveness of FTP/TPI. The frequency of KRAS mutations would have been interesting to explore because mOS and mPFS was independent of KRAS-status in RECOURSE and TERRA but highly dependent on KRAS-mutations in the Japanese phase II trial [Citation4].
FTD/TPI and regorafenib have not been compared directly in a clinical trial but in observational series, however, both the efficacy [Citation36] and the effectiveness seem comparable in third line therapy in patients with mCRC [Citation15,Citation16]. However, regorafenib has an entirely different mechanism of action and is associated with more toxicity than FTD/TPI [Citation1]. In the present study, approximately one in three patients had received both FTD/TPI and regorafenib compared to 17% in RECOURSE. This has likely no impact on our overall estimates of effectiveness since Cremolini et al. [Citation18] compared patients who had received both agents and reported that all outcomes were independent of the sequence.
Some additional limitations to our study need attention. We aimed at including real life patients. Nevertheless, our results may not fully reflect true real life conditions. Some of the included series are rather small with 14, 17, and 21 patients and were collected over a longer period. These patients may have been carefully selected and not true real-life patients. The bigger series are probably more representative for real life patients, but sometimes the compassionate use programs are very restricted, even more than the inclusion criteria to a phase III study.
All included studies are biased by the lack of controlled design and do not follow the intention-to-treat principle. Completeness of reporting was heterogeneous and not all included studies provided comprehensive baseline characteristics such as previous lines of therapy. Hence, we cannot exclude potential inconsistencies between the included studies. Finally, since six of the included studies were Japanese (n = 510 from 35 centres) and three were European (n = 498 from 29 centres), the relevance of our results for other populations is unknown. Still, data from the European and Japanese centres appeared comparable.
Given the modest survival effect, recognizing the patients who benefit from FTD/TPI is extremely important [Citation37]. The absence of valid predictive biomarkers leaves clinicians to select patients to FTD/TPI based primarily on prognostic considerations. This systematic review and a meta-analysis of real life studies allows us to conclude that when clinicians decide to prescribe FTD/TPI monotherapy as salvage-line treatment in patients with therapy refractory mCRC, they may reproduce the efficacy know from RECOURSE but not the survival benefit that was reported in the two Asian efficacy studies. Combination regimens may further improve the therapeutic yield of FTD/TPI and several clinical trials are underway [Citation38].
Supplemental Material
Download MS Word (88.1 KB)Acknowledgments
The authors would like to express their thanks to the departments of surgery and medical oncology at Izumi City General Hospital, Japan, and to the Danish regional drug and therapeutic committees for kindly providing data.
Disclosure statement
SEA, IBA, VBJ, OT, and JSL: None.
Additional information
Funding
References
- Sunakawa Y, Izawa N, Mizukami T, et al. Profile of trifluridine/tipiracil hydrochloride in the treatment of metastatic colorectal cancer: efficacy, safety, and place in therapy. Onco Targets Ther. 2017;10:4599–4605.
- Yoshino T, Uetake H, Fujita N, et al. TAS-102 safety in metastatic colorectal cancer: results from the first postmarketing surveillance study. Clin Colorectal Cancer. 2016;15:205–211.
- Mulet N, Matos I, Noguerido A, et al. Evaluating trifluridine + tipiracil hydrochloride in a fixed combination (TAS-102) for the treatment of colorectal cancer. Expert Opin Pharmacother. 2018;19:623–629.
- Yoshino T, Mizunuma N, Yamazaki K, et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2012;13:993–1001.
- Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–1919.
- Xu J, Kim TW, Shen L, et al. Results of a randomized, double-blind, placebo-controlled, phase III trial of trifluridine/tipiracil (TAS-102) monotherapy in asian patients with previously treated metastatic colorectal cancer: the TERRA study. J Clin Oncol. 2018;36:350–358.
- Streiner DL, Norman GR. Efficacy and effectiveness trials. Cumunity Oncol. 2009;6:472–474.
- Cohen AT, Goto S, Schreiber K, et al. Why do we need observational studies of everyday patients in the real-life setting? Eur Heart J Suppl. 2015;17:D2–D8.
- Joanna Briggs Institute. JBI Critical Appraisal Checklist for Cohort Studies. Avaiilable at http://joannabriggs.org/research/critical-appraisal-tools.html
- Arita S, Shirakawa T, Matsushita Y, et al. Efficacy and safety of TAS-102 in clinical practice of salvage chemotherapy for metastatic colorectal cancer. Anticancer Res. 2016;36:1959–1966.
- Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and forest plots using a microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes. 2012;5:52.
- Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13:152.
- Bland JM, Kerry SM. Statistics notes. Weighted comparison of means. Br Med J. 1998;316:129.
- Ota T, Tsukuda H, Hasegawa Y, et al. Treatment of TAS-102 in patients with metastatic colorectal cancer. Ann Oncol. 2016;27.
- Masuishi T, Taniguchi H, Hamauchi S, et al. Regorafenib versus trifluridine/tipiracil for refractory metastatic colorectal cancer: a retrospective comparison. Clin Colorectal Cancer. 2017;16:e15–e22.
- Moriwaki T, Fukuoka S, Taniguchi H, et al. Propensity score analysis of regorafenib versus trifluridine/tipiracil in patients with metastatic colorectal cancer refractory to standard chemotherapy (REGOTAS): a Japanese society for cancer of the colon and rectum multicenter observational study. Oncologist. 2018;23:7–15.
- Kwakman JJM, Vink G, Vestjens JH, et al. Feasibility and effectiveness of trifluridine/tipiracil in metastatic colorectal cancer: real-life data from The Netherlands. Int J Clin Oncol. 2018;23:482–489.
- Cremolini C, Rossini D, Martinelli E, et al. Trifluridine/tipiracil (TAS-102) in refractory metastatic colorectal cancer: a multicenter register in the frame of the Italian compassionate use program. Oncologist. 2018;23:1–10.
- Sueda T, Sakai D, Kudo T, et al. Efficacy and safety of regorafenib or TAS-102 in patients with metastatic colorectal cancer refractory to standard therapies. Anticancer Res. 2016;36:4299–4306.
- Kotani D, Shitara K, Kawazoe A, et al. Safety and efficacy of trifluridine/tipiracil monotherapy in clinical practice for patients with metastatic colorectal cancer: experience at a single institution. Clin Colorectal Cancer. 2016;15:e109–e115.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386.
- Favoriti P, Carbone G, Greco M, et al. Worldwide burden of colorectal cancer: a review. Updates Surg. 2016;68:7–11.
- Mercier J, Voutsadakis IA. A systematic review and meta-analysis of retrospective series of regorafenib for treatment of metastatic colorectal cancer. Anticancer Res. 2017;37:5925–5934.
- Gomez SL, O’Malley CD, Stroup A, et al. Longitudinal, population-based study of racial/ethnic differences in colorectal cancer survival: impact of neighborhood socioeconomic status, treatment and comorbidity. BMC Cancer. 2007;7:193.
- Cicero G, De Luca R, Dieli F. Progression-free survival as a surrogate endpoint of overall survival in patients with metastatic colorectal cancer. Onco Targets Ther. 2018;11:3059–3063.
- Panageas KS, Ben-Porat L, Dickler MN, et al. When you look matters: the effect of assessment schedule on progression-free survival. J Natl Cancer Inst. 2007;99:428–432.
- Michiels S, Piedbois P, Burdett S, et al. Meta-analysis when only the median survival times are known: a comparison with individual patient data results. Int J Technol Assess Health Care. 2005;21:119–125.
- Huang B, Kuan P-F. Comparison of the restricted mean survival time with the hazard ratio in superiority trials with a time-to-event end point. Pharm Stat. 2018;17:202–213.
- Trinquart L, Jacot J, Conner SC, et al. Comparison of treatment effects measured by the hazard ratio and by the ratio of restricted mean survival times in oncology randomized controlled trials. J Clin Oncol. 2016;34:1813–1819.
- A’Hern RP. Cancer biology and survival analysis in cancer trials: restricted mean survival time analysis versus hazard ratios. Clin Oncol. 2018;30:e75–e80.
- Lacas B, Bourhis J, Overgaard J, et al. Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta-analysis. Lancet Oncol. 2017;18:1221–1237.
- Bullement A, Underhill S, Fougeray R, et al. Cost-effectiveness of trifluridine/tipiracil for previously treated metastatic colorectal cancer in England and Wales. Clin Colorectal Cancer. 2018;17:e143–e151.
- Haynes B. Can it work? Does it work? Is it worth it? The testing of healthcareinterventions is evolving. Br Med J. 1999;319:652–653.
- Singal AG, Higgins PDR, Waljee AK. A primer on effectiveness and efficacy trials. Clin Transl Gastroenterol. 2014;5:e45.
- Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?” Lancet. 2005;365:82–93.
- Abrahao ABK, Ko Y-J, Berry S, et al. A comparison of regorafenib and TAS-102 for metastatic colorectal cancer: a systematic review and network meta-analysis. Clin Colorectal Cancer. 2018;17:113–120.
- van der Velden DL, Opdam FL, Opdam FL. TAS-102 and the quest for predictive biomarkers. ESMO Open. 2017;2:e000263.
- Peeters M, Cervantes A, Moreno VS, et al. Trifluridine/tipiracil: an emerging strategy for the management of gastrointestinal cancers. Future Oncol. 2018;14:1629–1645.