Socioeconomic inequalities in screening participation and awareness of early symptoms of cancer can accentuate unfair inequalities in clinical stage at diagnosis and prognosis among patients. Surveillance of sociodemographic disparities in early detection of cancer should rely on (i) data on clinical stage at diagnosis obtained from cancer/quality registers with high coverage, (ii) sociodemographic data on incident cases and the underlying population, obtained from total population registers and (iii) adequate statistics. Commonly, indicators of early detection are based on counts of patients only (numbers of patients with regard to stage). For example, in the Scottish Detect Cancer Early programme, the proportion of patients diagnosed with stage I disease has been monitored for breast, colorectal and lung cancers [Citation1]. However, any assessment of patterns in counts should also consider demographic variations within the underlying population at risk. For example, if the age distribution in a population varies over time, or between population groups, comparisons of counts are likely to be biased, because higher age is usually associated with later stage at diagnosis. Herein, we suggest an adequate basic statistic for surveillance of sociodemographic inequalities in early detection of cancer. Moreover, we suggest a feasible procedure for routine collection of sociodemographic data.
Cutaneous malignant melanoma as an example
Cutaneous malignant melanoma (CMM) has caught legitimate publicity around the world for continuously increasing incidence rates [Citation2]. There are several possible reasons behind the dramatic increase in CMM incidence observed in many countries, e.g., increased UV exposure, histopathological diagnostic drift, greater public awareness and introduction of screening activities [Citation3]. The two latter ones, and possibly also improved diagnostics, are expected to increase the number of tumours diagnosed early. The Swedish Melanoma Quality Register routinely reports the proportion of thin CMM tumours with thickness ≤1 mm, which conveys excellent prognosis. During recent years, this proportion has increased in Sweden [Citation4]. Besides, a steadily increasing time trend in the age-standardised incidence of thin CMM has been reported [Citation5].
Sociodemographic inequalities in early detection of CMM were addressed in a population-based study in the Southern and Western Swedish Health Care Regions, which showed that lower educational level was associated later stages at diagnosis [Citation6]. In that study, no analysis of time trends was performed. Incident CMM cases were identified from the Swedish Cancer Register. Data on clinical stage at diagnosis (I–IV) were obtained by linkage to the Swedish Melanoma Quality Register (>98% coverage) [Citation6]. According to the staging system of CMM used during the study period, stage I corresponded to all CMMs with a Breslow thickness ≤1.0 mm and CMMs with a 1.1–2.0 mm thickness without ulceration; stage II included CMMs of 1.1–2.0 mm thickness with ulceration and all CMMs >2.1 mm; stage III comprised CMMs with spreading to regional lymph nodes, whereas stage IV corresponded to CMMs with distant metastases. Data on educational level and country of birth were obtained by linkage to Statistics Sweden’s population registers. Cases were classified according to immigrant status (Swedish-born or Foreign-born) and in relation to the number of school years completed at the end of the year of diagnosis (‘low’ ≤9 years (primary school), ‘intermediate’ 10–12 years (high school/pre-university level) and ‘high’ ≥13 years (university level)). Corresponding sociodemographic data for the underlying population at risk, i.e., the study population size by calendar year, sex, 5-year age groups, educational level and immigrant status, were also obtained from Statistics Sweden. We underline that the sociodemographic data on the cases were collected for the purpose of performing a register-based study. Below, we suggest another approach to collecting sociodemographic data on incident cases – a more feasible procedure for the purpose of regular surveillance.
For our demonstration, cases with a diagnosis of a first primary invasive CMM between 1 January 2008 and 31 December 2016, were included (5209 men, 5098 women; data updated since reported in [Citation6]). We calculated, for each year, the age-standardised incidence of CMM by clinical stages I, II and III–IV, using the European standard population [Citation7]. shows the resulting time trends for men and women. The stage I CMM incidences increased markedly over time, while the variations in the incidences of later stage CMM were modest.
Figure 1. Time trends in the age-standardised, stage-specific incidences of CMM in men and women, respectively.
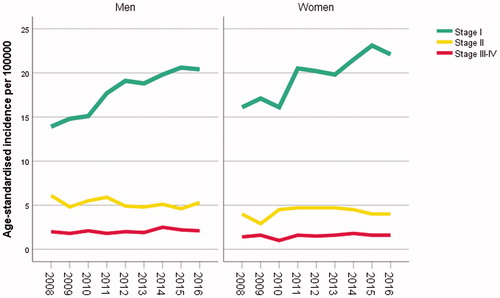
We considered eight groups of cases and corresponding population groups: Swedish-born men and Swedish-born women with high, intermediate and low educational level, respectively, Foreign-born men and Foreign-born women. Immigrants (7% of the cases) were not classified according to educational level because of insufficient data quality with considerable misclassification [Citation8]. The incidence of all-stage CMM showed an increasing time trend in each population group (Supplementary Figure). Immigrants had markedly lower incidence rates. Among the Swedish-born, the incidence was generally highest for people with high educational level.
We compared two basic statistics: (1) the proportion of cases with stages II–IV disease; denoted Pr{stages II–IV}. (2) The proportion of the age-standardised incidence attributed to stages II–IV disease, i.e., the ratio between the age-standardised incidence rate of stages II–IV cases and the age-standardised incidence rate of all cases; denoted PrASI{stages II–IV}. PrASI{stages II–IV} is thereby controlled for confounding by age, in contrast to the conventional Pr{stages II–IV}. We chose to combine stages II–IV because of fairly stable time trends of stage II and stages III–IV CMMs (). shows the values on these basic statistics, along with the fitted linear trends, for Swedish-born men/women with high compared to low educational level. These declining linear trends seem to be fairly parallel for men; and for women as well. For Swedish-born women, values on PrASI{stages II–IV} in the beginning (year 2008) were similar to the ones reached by the Swedish-born men in the end of the observation period. The other statistic, Pr{stages II–IV}, indicated upward biased disparities linked to educational level, because of confounding by age (elderly persons have lower educational level and later stages at diagnosis). Pr{stages II–IV} yielded, on average, 7 percentage points upward biased difference between low and high educational level for Swedish-born men; the corresponding bias was 14 percentage points for Swedish-born women. Regarding the groups not presented in , we point out that the fitted linear trends of PrASI{stages II–IV} for Swedish-born men/women with intermediate educational level lie in between the regression lines shown. Data on Foreign-born men and women yielded similar linear regressions of PrASI{stages II–IV} as those shown for Swedish-born men and women with low educational level.
Figure 2. The suggested basic statistics, PrASI{stages II–IV}, compared to the conventional one, Pr{stages II–IV}. The calculated values on these statistics and corresponding linear regression lines are shown for Swedish-born men with high and low educational level, and for in Swedish-born women with high and low educational level.
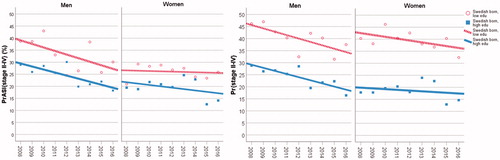
Taken together, the descriptive results suggest a decline in the relative frequency of stages II–IV CMM cases in each group, mainly due to increasing incidences of stage I CMM. Poisson regression analyses of the stage-specific counts on the covariates year (categorical), age (categorical, 5-year groups) and population group (eight groups), implied that the time trend for stage I CMM was significant (p < .001), whereas the time trends for stage II CMM (p = .22) and stages III and IV CMM (p = .15) were not significant. Furthermore, group affiliation did not evidently modify the overall stage-specific time patterns (Wald’s test for the interaction term year×group: stage I, p = .45; stage II, p = .62; stages III and IV, p = .99). These analytic results indicate that the sociodemographic disparities in early detection of CMM have not changed notably (with regard to the multiplicative scale) during the observation period 2008–2016.
The proposed PrASI statistic is a relevant descriptive statistic for surveillance, which helps to fine-tune further analyses of sociodemographic inequalities in the early detection of CMM. However, using PrASI as a quality indicator, rather than the proportion of CMM patients with thin melanomas [Citation9], may yet be problematic due to possible, parallel trends in overdiagnosis. In fact, the incidence of CMM in situ in Sweden has shown a dramatic increase during the past decades and the proportion of CMM in situ among all diagnosed CMMs has increased from approximately 25–45% between the late 1990s and 2017 [Citation9]. Analysis of CMM in situ data could provide further insights into the overdiagnosis issue. Yet, data may be unreliable due to the poor inter- and intra-observer diagnosis among pathologists [Citation10].
A procedure for routine collection of sociodemographic data
We suggest that incident cancer cases should be routinely geo-coded into adequately small areas based on residential address, in order to use sociodemographic characteristics on area-level [Citation11]. In Sweden, the current geo-coding of cancer cases into municipalities is too crude. Statistics Sweden has recently established Demographic Statistics Areas (denoted ‘DeSO’) within each municipality in Sweden [Citation12]. In total, there are almost 6000 such areas, currently with population sizes varying between 700 and 2700 persons. These geographical areas will not be changed in the future and they provide an opportunity for new geo-coding of incident cancer cases in Sweden, with accessible area-level data on sociodemographic characteristics. Selected area-level characteristics may be used for classifying cases and underlying population into sociodemographic strata according to proportion of residents with low educational level, proportion of households with low purchasing power, proportion of immigrants, etc., provided by residential DeSO. In addition, such geo-coding facilitates surveillance of stage-specific incidence patterns in time and space, by using geographically structured models [Citation13].
Ethical approval
The data used for demonstration were obtained from a study which was approved by the Regional Ethical Review Board in Lund, Sweden.
Supplemental Material
Download TIFF Image (1.2 MB)Acknowledgments
We thank Stefan Peterson, Anders Holmén and Erik Holmberg for work with data. This article was prepared during the first author’s sabbatical at the School of Public Health, Imperial College London, which was supported by the Wenner-Gren Foundations.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- National Services Scotland, Information Services Division. Detect cancer early staging data; 2017. Available from: https://www.isdscotland.org/Health-Topics/Cancer/Publications/2017-07-25/2017-07-25-DetectCancerEarly-Report.pdf?8512514830
- Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol. 2016;136:1161–1171.
- Gardner LJ, Strunck JL, Wu YP, et al. Current controversies in early-stage melanoma: questions on incidence, screening, and histologic regression. J Am Acad Dermatol. 2019;80:1–12.
- Ingvar C, Eriksson H. Every second hour there is a new melanoma diagnosed in Sweden. Lakartidningen. 2017;114. pii: ELAT.
- Claeson M, Gillstedt M, Whiteman DC, et al. Lethal melanomas: a population-based registry study in Western Sweden from 1990 to 2014. Acta Derm Venereol. 2017;97:1206–1211.
- Strömberg U, Peterson S, Holmberg E, et al. Cutaneous malignant melanoma show geographic and socioeconomic disparities in stage at diagnosis and excess mortality. Acta Oncol. 2016;55:993–1000.
- NORDCAN – Cancer statistics for the Nordic countries. Available from: http://www-dep.iarc.fr/NORDCAN/english/glossary.htm
- Saarela J, Weber R. Assessment of educational misclassification in register-based data on Finnish immigrants in Sweden. Scand J Public Health. 2017;45:20–24.
- Swedish Melanoma Study Group. Swedish Melanoma Registry. Quality report for the diagnosis year 1990–2017. Available from: https://www.cancercentrum.se/globalassets/om-rcc/sydost/pdf/nationell-kvalitetsregisterrapport-hudmelanom-1990-2017.pdf
- Elmore JG, Barnhill RL, Elder DE, et al. Pathologists' diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ. 2017;357:j2813.
- Tweed EJ, Allardice GM, McLoone P, et al. Socio-economic inequalities in the incidence of four common cancers: a population-based registry study. Public Health. 2018;154:1–10.
- Statistics Sweden. [Att mäta segregation på låg regional nivå. Slutrapportering av uppdrag till Statistiska centralbyrån att genomföra en förstudie om rikstäckande områdes indelning för statistisk uppföljning av socioekonomiska förhållanden]. Report 2017/1421.
- Boulieri A, Bennett JE, Blangiardo M. A Bayesian mixture modeling approach for public health surveillance. Biostatistics. 2018 Sep 25. doi: 10.1093/biostatistics/k y/038. [Epub ahead of print].
