Abstract
Background: Fatigue is a common and distressing cancer symptom that negatively affects the quality of life. Many scales have been developed to assess cancer-related fatigue. The properties of the scales vary in terms of dimensionality, reliability, validity, length and method of administration. Insufficient of psychometric properties may affect the accuracy of scales findings, that may lead result obtained questionable. The main objective of this review was to conduct a quality assessment of the psychometric properties of cancer-related fatigue scales to identify appropriate scales that could be used in research and clinical practice.
Method: A systematic search was carried out to identify validated scales that measure cancer-related fatigue. Five databases were searched: CINAHL, MEDLINE, EMBASE, PsycINFO, Cochrane Library. This review was conducted following the PRISMA and Terwee et al.’s quality assessment guidelines to evaluate the psychometric properties of the studies.
Result: Seventy-one different studies published between 1970 and 2018 met the inclusion criteria. Twenty-five scales were identified. Of these, eighteen were multidimensional and seven were uni-dimensional, containing between 4 and 72 items. Reliability and/or validity information was missing for many scales. Four scales met the quality assessment criteria and were reported as the most appropriate for measuring fatigue in cancer patients.
Conclusion: Further psychometric testing is required for other scales. Developing a universally-defined tool kit for the assessment of cancer-related fatigue may help clarify the concept of fatigue and promote a systematic approach to fatigue measurement.
Introduction
A cancer diagnosis is a major life stressor that affects an individual’s physiological and psychological state. Patients may experience symptoms related to their cancer and/or cancer treatment with fatigue being a common and distressing symptom. Estimated prevalence rates range from 50% to 90% [Citation1]. The variation in prevalence among similar cancer patient populations may be partially dependent upon how cancer-related fatigue (CRF) is measured. Many cancer patients described fatigue highly distressing symptom affecting their quality of life [Citation2,Citation3]. Fatigue occurs during cancer diagnosis, treatment and throughout the survival trajectory [Citation4] including long-term disease-free survivors [Citation5], and advanced cancer patients [Citation1]. CRF typically increases during radiation [Citation6], chemotherapy [Citation7] and biological therapy [Citation8].
Despite the prevalence of CRF, there is no universally agreed upon definition of CRF or gold standard questionnaire to measure this troubling symptom [Citation9]. One of the most commonly cited definitions, proposed by the National Comprehensive Cancer Network (NCCN), states that fatigue is ‘a distressing persistent subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity that interferes with usual functioning’ [Citation10]. The European Association for Palliative Care (EAPC) defined CRF as a ‘subjective feeling of tiredness, weakness or lack of energy’ [Citation11]. Both definitions described CRF as subjective, indicating that the assessment of fatigue should be solicited directly from the individual. One difference between the EAPC and NCCN definitions is the impact of CRF on functioning, with the latter being more comprehensive to include the consequences of fatigue on function. The conceptual definition of CRF should guide the operationalization of outcome measures used in research studies. Consequently, the different CRF definitions employed within research studies may help explain the variety of scales used to measure fatigue.
Researchers strive to use CRF scales with acceptable reliability and validity. Insufficient reporting or testing of psychometric properties may affect the accuracy of findings, leading to questionable study results [Citation12–14]. The use of valid and reliable scales to measure CRF may lead to an improvement in patient care and the development of fatigue guidelines. Four previous systematic reviews have been published which explore the reliability and validity of CRF measuring scales [Citation15–18]. Whilst such reviews were helpful, they did not employ any structured quality assessment criteria to determine the psychometric properties of the scales being reviewed. Terwee et al. [Citation19] provided researchers with quality assessment criteria to evaluate studies that have reported the psychometric properties of questionnaires. Hence, the purpose of this review is to assess the psychometric properties of CRF scales using the Terwee et al.’s quality assessment criteria.
Methods
Search strategy
A systematic literature search was conducted using the following electronic databases: CINAHL, MEDLINE, EMBASE, PsycINFO, Cochrane Library, for the period from 1946 to December 2018. The search strategy used in each database was as follows: (MH ‘Patient Assessment+’) OR (MH ‘Clinical Assessment Tools+’) OR (MH ‘Functional Assessment+’) OR (MH ‘Outcome Assessment’); (MH ‘Outcome Assessment’) OR (MH ‘Measurement Issues and Assessments+’); (MH ‘Inventories’); (MH ‘Scales’); (MH ‘Cancer Fatigue’) OR (MH ‘Fatigue Syndrome, Chronic’); (fatigue adj (scale or inventory or instrument or measurement or assessment)).mp.; (MH ‘Process Assessment (Health Care)+’); (MH ‘Clinical Assessment Tools+’); (MH ‘Measurement Issues and Assessments+’) OR (MH ‘Psychometrics’); (MH ‘Fatigue+’); (MH ‘Hematologic Neoplasms+’); (MH ‘Palliative Care’); Neoplasm*; (MH ‘Cancer Fatigue’) OR (MH ‘Neoplasms+’). Footnote chasing was used to identify any further studies [Citation20].
Selection criteria
Studies published in English with a stated study purpose to evaluate the psychometric properties of a CRF instrument were eligible for inclusion. People with cancer were the population of interest. Studies were included in the review if study participants met the following inclusion criteria: (1) aged 18 or more; (2) diagnosed with any type of cancer; (3) any stage of cancer; (4) in the case of mixed patient populations, studies were included if more than half of the participants were diagnosed with cancer. Exclusion criteria were protocol papers and conference abstracts; single-item scales such as visual analog scales (VAS) and fatigue subscales that were part of quality of life scales.
Assessment of measurement properties of CRF Scales
Upon retrieval of applicable studies, CRF scales were evaluated to determine dimensionality, (unidimensional that produces one overall fatigue score versus multidimensional that produces subscales score with or without an overall fatigue score), number of items and method of administration. The psychometric properties of CRF scales of studies included in the review were assessed using Terwee et al.’s [Citation19] quality assessment criteria (Supplementary Table S1). These criteria specifically address eight measurement properties: content validity, internal consistency, criterion validity, construct validity, reproducibility, responsiveness, floor and ceiling effects, and interpretability. Using the descriptors provided by Terwee et al. [Citation19], the eight criteria were applied to each CRF scales within the review and rated as positive (+); indeterminate (?); negative (−); or ‘0’, no information available.
For ease of interpretation, this review is reported in two sections. Section one: addresses Terwee et al.’s [Citation19] quality assessment criteria to evaluate each study’s report of the CRF scales. Section two: reviewed the scales’ characteristics, such as the number of items, dimensions of fatigue, scoring system, administration time, the reporting period for the fatigue assessed, and the quality assessment of the scales. Unidimensional and multidimensional scales are presented separately.
Findings
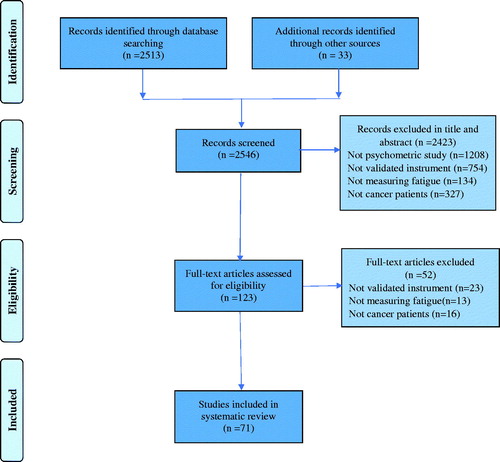
The search strategy identified 2546 studies, which were screened by abstract and title. Based on the inclusion/exclusion criteria, 71 studies employing 25 different scales were included in the review (). There were 7 unidimensional and 18 multidimensional scales; the former produced an overall fatigue score, whereas the multidimensional scales produced subscale scores and an overall CRF score.
The review evaluated 71 studies with five studies reporting more than one scale: four studies evaluated two scales [Citation21–24] and one study evaluated three scales [Citation25]. Each scale in each study was evaluated separately. To account for studies that evaluated multiple CRF scales, the denominator for reporting percentages was 77 studies rather than 71 (66 studies reporting the psychometric properties of one CRF scales, 4 studies reporting two scales and one study reporting three scales).
Seventy-one psychometric studies involving 17,794 mixed cancer patients were included in this review (See Supplementary Table S2 for a general characteristic of the studies). The sample size ranged from 120 to 800. Study participants were diagnosed with a variety of cancers. The vast majority (n = 60 studies) included those with mixed cancer diagnoses. The remaining studies included women with breast cancer, Hodgkin’s lymphoma, prostate, head and neck, and lung cancers. Nineteen different definitions of CRF were identified across the studies however only 32 studies (41.5%) included a definition of CRF. The remaining studies stated that there was no universal definition of CRF. A summary of all definitions used in the studies is presented in Supplementary Table S3.
Section one: overall reporting of quality assessment criteria of CRF Scales
Psychometric properties were assessed for each CRF instrument based on the Terwee et al. [Citation19], checklist (see for a summary of the evaluation). Seventy studies (91%) received a positive rating in terms of content validity; six (7.8%) were indeterminate. Only one study [Citation26] provided no information about content validity. Internal consistency was reported in 71 studies (92%); 68 received a positive rating (88%) and three had an indeterminate rating (4%) [Citation27–29]. Sixty-two studies received a positive score (80.5%) for construct validity, while eight received an indeterminate score (10%) [Citation30–37]. However, six studies provided no information on internal consistency [Citation26,Citation38–42] and one study received a negative rating in terms of construct validity [Citation38] .
Table 1. The assessment of measurement properties of CRF scale (N = 77).
Studies evaluated other criterion less frequently. Only 23 studies (29.8%) provided information about criterion validity. Of these, 16 studies demonstrated positive (21%) criterion validity [Citation21,Citation27,Citation33,Citation38,Citation42–52] and just seven (9%) received an indeterminate rating [Citation37,Citation53–58]. The remaining 54 (70%) studies did not provide information related to criterion validity. Agreement was only assessed in two studies (2.5%) [Citation26,Citation59], which indicated a positive score for the instrument to detect minimally important changes in CRF. Reliability data were reported as being acceptable (i.e., at least 0.07) in 26 (34%) studies. Four studies produced an indeterminate rating (5%), and 47 (61%) studies did not provide any information. Responsiveness was evaluated in five studies (6.5%). One (1.3%) study received a positive rating for responsiveness [Citation29], while four studies (5.2%) were rated as indeterminate [Citation22,Citation60,Citation61]. Floor and ceiling effects were reported in five studies (6.5%); four receiving positive ratings (5.2%) [Citation23,Citation62,Citation63] and one (1.3%) an indeterminate rating [Citation32]. Three studies (3.9%) reported interpretation. Two (2.6%) received an indeterminate rating [Citation32,Citation64] and one (1.3%) a positive rating [Citation65].
Section two: scale characteristics
The review found seven different unidimensional scales to assess CRF (Supplementary Table S4).
Unidimensional Scales: Twenty-six studies examined the psychometric properties of unidimensional scales: (a) ten explored the Brief Fatigue Inventory (BFI) scale; (b) eight used the Functional Assessment of Chronic Therapy-Fatigue subscale (FACT-F); (c) three studies assessed the Fatigue Severity Scale (FSS) scales; (d) two studies evaluated the Modified Brief Fatigue Inventory (MBFI) and (e) one study measured Four-Items Fatigue Scale (FIFS), Fatigue Assessment Scale (FAS) and Fatigue Items Bank (FIB).
The BFI is a measure of fatigue severity for cancer populations [Citation53]. It consists of 9 items using a 11-point numerical rating scale. The original version was published in English. The reliability and validity were established in oncology outpatients, inpatients and healthy populations. The internal consistency (0.96) supports the reliability of the tool [Citation53]. The BFI is quick and easy for participants to complete. This inventory has been translated into a range of languages, including Italian [Citation43], Greek [Citation33], German [Citation44], Taiwan-Chinese [Citation64], Chinese [Citation66], Japanese [Citation45], Korean [Citation37], Indonesian [Citation67] and Filipino [Citation39]. The internal consistency in all translated versions of the BFI was high (between 0.96 and 0.91) and all versions were validated for use in mixed cancer populations. The method of translation from the original version of BFI to other languages was done according to the forward-backward procedures. In people with cancer, the BFI meets the quality assessment criteria for content, criterion and construct validity along with internal consistency and interpretation. Further work is needed with regard to agreement, responsiveness, and floor and ceiling effects.
The scoring system of the BFI was modified (MBFI) from the original 0–10 point numeric scale to a 1–7 point scale [Citation68]. The same 9 items were retained and validated in patients with head and neck cancer [Citation19]. Two validation studies of the MBFI, showed good internal consistency of the subscales (coefficient alpha: 0.93–0.86) [Citation21,Citation68]. In people with cancer, the MBFI meets the quality assessment criteria for content, criterion and construct validity along with reliability and internal consistency. However, agreement, responsiveness, floor and ceiling effects, and interpretation has not been reported.
The 9 items of BFI were reduced to 4 to develop the FIFS [Citation38]. It was tested in patients with different types of cancer. The FIFS did not predict fatigue over time and the reliability of the scale needs to be confirmed in further studies. In people with cancer, the FIFS meets the quality assessment criteria for content and criterion validity. Reliability, internal consistency agreement, responsiveness, floor and ceiling effects, and interpretation have yet to be reported.
The FACT-F is a 13-item questionnaire using a 5-point Likert scale to assess fatigue [Citation46]. It has been validated for use with a variety of cancer diagnoses and treatments [Citation46]. The original FACT-F showed strong internal consistency (coefficient alpha: 0.93–0.95) and good stability (test-retest, r = 0.87) [Citation46]. The FACT-F has been translated in to 57 languages using iterative forward-backward translation methodology [Citation69]. Eden and Kunkel [Citation21] validated the FACT-F with patients with head and neck cancer and reported good internal consistency (Cronbach’s alpha: 0.87) and test-retest reliability (r = 0.95). Other psychometric studies using the instrument translated it into Spanish [Citation61], French and Dutch [Citation70], Japanese [Citation40], Persian [Citation71] and Portuguese [Citation26,Citation72]. Internal consistency of the translated scales ranged from between 0.79 and 0.94. In people with cancer, the FACT-F meets the quality assessment criteria for content, criterion and construct validity along with agreement, reliability and internal consistency. Further work, however, is needed on responsiveness, floor and ceiling effects, and interpretation.
The FAS includes 10-items and uses a 5-point Likert scale to assess physical and mental fatigue. Even though the FAS assesses two dimensions of fatigue, it is categorized as unidimensional as only the overall fatigue score should be used [Citation73,Citation74]. De Vries et al. [Citation75] assessed the psychometric properties in a working population of 560 Dutch breast cancer patients; the results showed good internal consistency (Cronbach’s alpha: 0.89) and test-retest reliability (r = 0.88). The FAS meets the quality assessment criteria for content and construct validity along with internal consistency and reliability. Further work is needed on criterion validity, agreement, responsiveness, floor and ceiling effects, and interpretation.
The FSS includes 9 items and uses a 7-point Likert scale. The FSS was originally validated in multiple sclerosis and systemic lupus erythematosus populations [Citation76]. The psychometric properties were assessed in advanced cancer patients [Citation54] and mixed cancer patients [Citation28]. The coefficient alpha of the two studies ranged from 0.94 to 0.96. Based on the review findings, the FSS meets the quality assessment criteria for content, criterion, and construct validity along with reliability, internal consistency and interpretation. Agreement, responsiveness and floor and ceiling effects have not been reported.
The FIB is a 72-item, 5-point Likert scale which was developed to measure CRF in a computerized adaptive testing format [Citation63]. The scale was validated in 301 mixed cancer patients. The FIB shows good psychometric properties using Rasch analysis. The internal consistency was 0.99 and item-total correlation was between 0.51 and 0.85. The factor analysis confirmed that 72 items were unidimensional. The authors provided a 6-item short form FIB for use in a clinical setting. In people with cancer, the FIB-72 items met the quality assessment criteria for content and construct validity, floor and ceiling effects along with internal consistency. Further work is needed on criterion validity, agreement, reliability, interpretation and responsiveness.
Multidimensional Scales: The review found 51 studies that investigated the properties of 18 multidimensional scales of fatigue. All 18 multidimensional scales provided an overall fatigue score, as well as subscale scores to represent specific domains of fatigue (Supplementary Table S5 for more details).
The Multidimensional Fatigue Inventory (MFI) is a 20-item, 5-point Likert scale designed to measure general fatigue, physical fatigue, mental fatigue, reduced motivation and reduced activity [Citation77]. The scale was originally validated in Dutch cancer patients, non-cancer chronic fatigue syndrome patients, army recruits and medical students [Citation77]. Smets et al. [Citation78] validated the MFI-20 in Dutch and Scottish patients with cancer and reported good internal consistency (coefficient alph: 0.79–0.93). The MFI-20 has been translated into several languages; French [Citation56], Chinese [Citation57], Brazilian Portuguese [Citation79], Polish [Citation55], Hindi [Citation80] and Swedish [Citation81], utilizing the back-translation process. Overall, internal consistency was acceptable in all translated versions of the MFI (between 0.80 and 0.90). In people with cancer, the MFI-20 meets the quality assessment criteria for content, criterion, and construct validity along with internal consistency and interpretation. Further work is needed on test-retested reliability, agreement, responsiveness, and floor and ceiling effects.
The Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF) is a 30-item, 5-point Likert scale that was designed specifically for use with breast cancer patients [Citation82]. There are 5 subscales; physical, emotional, mental, vigor and general fatigue. Initial psychometric testing occurred in 224 breast cancer patients, who were undergoing chemotherapy and radiotherapy, and the scale demonstrated very good validity and reliability [Citation82]. Additional psychometric studies were conducted in English [Citation83], Chinese [Citation84] and Singapore Chinese [Citation60], and the internal consistency of the subscales ranged from 0.74 to 0.96. In people with cancer, the MFSI-SF met the quality assessment criteria for content and construct validity along with internal consistency, reliability and responsiveness. Criterion validity, agreement, floor and ceiling effects, and interpretation were not reported.
Schwartz [Citation85] published the first version of the Schwartz Cancer Fatigue Scale (SCFS), which has 28-items using a 5-point Likert scale. The SCFS assess four fatigue dimensions; physical, cognitive, temporal and emotional, and was specifically designed to measure CRF. The coefficient alpha for the total scale score was 0.96, and subscales ranged from 0.82 to 0.93. A revised version, the SCFS-6 contains only 6-items, measuring the physical and perceptual dimensions of CRF and has shown good internal consistency (coefficient alpha: 0.81–0.88) [Citation86] and 0.85 [Citation29]. The SCFS-6 was translated into Chinese and had good internal consistency (coefficient alpha: 0.88–0.89) [Citation23,Citation25] using backward transitional methods. In people with cancer, the SCFS-6 met the quality assessment criteria for content and construct validity along with reliability, internal consistency, floor and ceiling effects, and responsiveness. Further work is needed on criterion validity, agreement and interpretation.
The Fatigue Symptom Inventory (FSI) consists of 13 items that assess the intensity, duration, daily pattern and interference of fatigue [Citation87]. The FSI was originally developed using a sample of patients with breast cancer, both during and after treatment. The internal consistency of the subscales had a coefficient alpha above 0.90 in all groups. Hann et al. [Citation88] tested FSI in a mixed cancer population and found an overall coefficient alpha of 0.94. The FSI was translated into Chinese and had a Cronbach’s alpha score of between 0.70 and 0.90 [Citation23,Citation25]. In people with cancer, the FSI met the quality assessment criteria for content, criteria and construct validity as well as reliability, internal consistency, and floor and ceiling effects. Agreement, responsiveness and interpretation have not been assessed.
The original Piper Fatigue Scale consisted of 40 items [Citation89]; reduced to 22 items in the revised version (PFS-R) [Citation34]. The revised version measured four subscales: behavioral/severity, affective meaning, sensory and cognitive/mood. The scales were validated in 382 breast cancer survivors. Internal consistency was high, over 0.90. The PFS-R has been validated in eight languages. Two psychometric studies were conducted in Italian [Citation30,Citation48]. Other validation studies were performed in Spanish [Citation41], Swedish [Citation27,Citation90], Dutch [Citation47], Portuguese [Citation91], Chinese [Citation35] and Korean [Citation31]. All translated versions of the PFS-R showed good internal consistency. A further reduction in the PFS items was carried out to create a 12-item scale (PFS-12) [Citation92], with the reliability of the item subscales being between 0.87 and 0.98. The PFS-12 measured four subscales: behavioral, affective, sensory and cognitive/mood. In people with cancer, the PFS-R met the quality assessment criteria for content, construct and criterion validity along with reliability and internal consistency. Further work is needed on agreement, responsiveness, floor and ceiling effects, and interpretation.
The Perform Questionnaire (PQ) [Citation62] consists of 12 items and was originally developed to measure fatigue among Spanish-speaking cancer patients. The scale assesses three dimensions: physical limitations, activities of daily living, and beliefs and attitudes. The scale was validated with a 238 mixed cancer patient population receiving adjuvant treatment, curative treatment and palliative care. The internal consistency ranged from 0.78 to 0.92. Another psychometric study conducted by Baró et al. [Citation65] found an overall internal consistency of 0.94 and test-retest reliability of 0.83. The PQ had good validity and reliability but was only validated in Spanish. In people with cancer, the PQ meets the quality assessment criteria for content, and construct validity along with reliability, internal consistency, interpretation, and floor and ceiling effects. Further work is needed on criterion validity, agreement and responsiveness.
The Lee Fatigue Scales (LFS) is an 18-item scale, which was originally developed to measure fatigue in patients with sleep disorders and is also known as the Visual Analog Scale for Fatigue, (VASF) [Citation93]. The LFS has two subscales: fatigue (13 items) and energy (5 Items). The psychometric properties in cancer patients were assessed by Meek et al. [Citation22]. The scale demonstrated good reliability but low stability because of sensitivity to morning and evening changes [Citation22]. Lerdal et al. [Citation32] evaluated the psychometric properties of the 13 fatigue item subscale of the LFS in 587 mixed cancer patients. Pearson’s correlation coefficients of the LFS were deemed acceptable (test-retest: r = 0.88). In people with cancer, the LFS 13-items met the quality assessment criteria for content and construct validity along with internal consistency and responsiveness. Further work is needed on criterion validity, agreement, reliability, floor and ceiling effects, and interpretation.
The Multidimensional Assessment of Fatigue (MAF) consists of 16 items scored on a scale ranging from 0 to 10 and originally validated in rheumatoid arthritis patients [Citation94]. The MAF has four dimensions of fatigue: severity, distress and degree of interference in activity of daily living, and timing. Winstead-Fry [Citation24], tested the MAF in a mixed cancer patient population and found adequate internal consistency. Additional psychometric testing was carried out in a cancer population by Meek et al. [Citation22] who reported the overall coefficient alpha to be 0.88. Despite this, the MAF failed to show adequate construct validity in terms of a four-factor structure [Citation22]. Several studies did not recommend using MAF unless further validation has been performed in the cancer population [Citation15,Citation17]. In people with cancer, the MAF met the quality assessment criteria for content validity as well as internal consistency. Further testing of criterion and construct validity, reliability, agreement, responsiveness, floor and ceiling effects, and interpretation are required.
The Cancer Fatigue Scale (CFS) is a 15-item, 5-point Likert scale composed of three domains: physical, affective and cognitive [Citation58]. It was validated in Japanese cancer patients. The reliability coefficients ranged from between 0.84 and 0.88. The construct validity showed a good score for the instrument (0.32–0.67). Okuyama et al. [Citation95] tested the CFS in breast cancer patients and found Cronbach’s alpha coefficients ranged from 0.76 to 0.89. Validation was completed for the Japanese version only. The CFS was translated into English but not tested psychometrically. Other translations include Chinese [Citation25], Dutch [Citation96], Turkish [Citation52] and Greek [Citation49] with good reliability (0.74–0.91). Based on the review findings, the CFS met the quality assessment criteria for content, construct and criterion validity as well as internal consistency. Further work is needed on agreement, interpretation, floor and ceiling effects, and responsiveness.
The Hirai Cancer Fatigue Scale (HCFS) is a 15-item, 5-point Likert scale, with three subscales; physical, mental and cognitive fatigue [Citation50]. The psychometric properties were assessed in a mixed cancer population of 281 patients undergoing treatment. The overall Cronbach’s alpha coefficient was 0.94, supporting internal consistency and a test-retest reliability coefficient of 0.82. The HCFS had high reliability and high validity based on the Japanese cancer population, but further validation is needed for the English version. In people with cancer, the HCFS met the quality assessment criteria for content, criterion and, construct validity as well as internal consistency and reliability in a Japanese population. Further work is needed on agreement, responsiveness, floor and ceiling effects, and interpretation.
The Cancer-Related Fatigue Distress Scale (CRFDS) is a 20-item scale that was originally investigated in a heterogeneous cancer population [Citation59]. The scale used an 11-point numerical rating scale to assess five domains; physical, social, psychological, cognitive and spiritual fatigue. The scale has very good validity and reliability. The internal consistency reliability was 0.98. In people with cancer, the CRFDS met the quality assessment criteria for content and construct validity as well as internal consistency. The criterion validity, reliability, responsiveness, floor and ceiling effects, and interpretation were not reported.
The Swedish Occupational Fatigue Inventory (SOFI) is a 25-item scale that was originally validated in work-related fatigue studies [Citation36]. The scale measures five fatigue domains: lack of energy, physical exertion, physical discomfort, lack of motivation and sleepiness. The SOFI was validated in a mixed Swedish cancer population of 81 patients receiving radiotherapy. The SOFI met the quality assessment criteria for content and criterion as well as internal consistency, interpretation, and floor and ceiling effects. Further work is needed on criterion and construct validity, reliability, agreement, responsiveness, floor and ceiling effects, and interpretation. Further psychometric analyses are required with a larger sample size.
The Wu Cancer Fatigue Scale (WCFS) has two versions. The original consists of 16 items with a 5-point Likert scale designed to assess physical, emotional and cognitive fatigue [Citation42]. It was tested in women with breast cancer undergoing chemotherapy. The scale shows adequate reliability and criterion-related validity. The three-factor model was not supported by exploratory factor analysis. The items in the revised WCFS-9 were reduced to 9 items [Citation51]. In people with cancer, the WCFS-9 meets the quality assessment criteria for content, criterion, and construct validity along with internal consistency. Further work is needed on agreement, reliability, responsiveness and floor and ceiling effects, and interpretation.
The Fatigue Functional Impact Scale (FFIS) was constructed to assess fatigue and functional impairment in an 8-item, 10-point Likert scale [Citation97]. The psychometric properties were assessed in 1355 mixed cancer patients receiving chemotherapy. The FFIS showed strong internal consistency with coefficient alpha scores of 0.90. In people with cancer, the FFIS met the quality assessment criteria for content and construct validity along with internal consistency. Criterion validity, agreement, reliability, floor and ceiling effects, interpretation and responsiveness were not assessed.
The General Fatigue Scale (GFS) is a 7-item scale that assesses overall fatigue intensity, distress level and disruptions of daily activity, and was designed to use for randomized controlled trials to measure fatigue at specific time-points [Citation98]. The scale was validated and translated into Chinese [Citation98]; the Cronbach’s alpha reliability coefficient was 0.94. In people with cancer, the GFS met the quality assessment criteria for content validity, construct validity, internal consistency and reliability. Criterion validity, agreement, floor and ceiling effects, responsiveness and interpretation were not reported.
Discussion
Assessing the impact of fatigue on people with cancer is imperative for understanding this distressing symptom and necessary for determining the impact of interventions on CRF. This review is the first to perform a quality assessment of CRF scales employing Terwee’s criteria of content validity, internal consistency, criterion validity, construct validity, reproducibility, responsiveness, floor and ceiling effects, and interpretability [Citation19]. Twenty-five scales assessed fatigue in adult cancer patients, considerably more than earlier reviews [Citation15,Citation17,Citation18,Citation99]. The quality assessment of CRF indicated that content, criterion and construct validity along with internal consistency were the most frequently met criteria, demonstrating that the vast majority of scales undergo at least some psychometric testing of validity and internal consistency reliability. On the other hand, reproducibility, in the form of agreement and intraclass correlation reliability, responsiveness, floor and ceiling effects, and interpretability are generally not assessed and/or reported as part of the psychometric testing. No study explored all of the Terwee et al. [Citation19] quality assessment criteria in evaluating the psychometric properties of CRF scales.
The use of Terwee et al.’s [Citation19] guidelines for assessing the psychometric properties of CRF scales allows users to distinguish between and make a judgment about the most appropriate choice of CRF scale. While each individual quality assessment criterion is important, the Terwee assessment does not result in an overall psychometric rating of a scale; thus, avoiding the assumption that all of the quality assessment criteria are equal. Terwee et al. [Citation19] suggested that the most important criteria of the nine measurement properties were content validity, as the absence of content validity can impact all other measurement properties [Citation100]. All of the scales in this review met the criterion for content validity; however, this indicator on its own, may not be helpful to researchers in selecting a CRF instrument.
Although other reviews of CRF scales have been conducted [Citation15–18], this current psychometric review of CRF scales adds to the literature. This review used different search terms and inclusion criteria and the search was conducted in additional databases compared to the previous reviews [Citation15–18]. As a result, this review included more studies by using different Medical Subject Headings (MeSH) terms and databases. This review evaluated scales using the Terwee et al. [Citation19] checklist for critical evaluation of psychometric properties for each of the studies that evaluated CRF scales. Hence, the current review adds new evidence by presenting each scale for CRF using well-defined criteria of psychometric properties. The most comprehensively validated scales were the BFI, FACT-F, MFI-20 and PFS-R. These received positive ratings in content validity, internal consistency, criterion validity, construct validity and reliability. In addition, these scales have been translated and psychometrically tested in different languages; adding to their value for use in people with cancer.
There are several other factors that need to be taken into consideration by researchers and clinicians when choosing a CRF scale. There is a need to clearly understand the phenomenon of CRF. Unfortunately, the lack of a consensus definition has led to the development of multiple scales assessing various dimensions or domains of CRF phenomena [Citation9]. It is imperative that the conceptual definition of fatigue is logically consistent with the operationalization of the concept. The CRF scales assessed within this review have different foci (e.g., some addressed fatigue severity while others assessed impact on function). The focus of the CRF instrument should be consistent with the research question. In addition, the heterogeneity of the type, the stage of cancer, treatment and type of management may entail different fatigue experiences, in terms of precedence and severity; thus, selection of a CRF scale should consider these factors. For example, the population in which the scale was validated previously should be taken into consideration, as cultural contexts and beliefs may impact on suitability or applicability of scale items.
There are few limitations to this review. Although this review included 71 studies using different Medical Subject Headings (MeSH) terms and databases, it is possible that some psychometrics studies were not included. This review was restricted to studies published in English. Other psychometric studies evaluating CRF scales published in a language other than English were not included in this review.
Conclusion
This review identified seven scales that produce an overall fatigue score and eighteen scales that produce fatigue subscale score with or without an overall fatigue scale score. These scales were drawn from 77 studies assessing fatigue in people with cancer. This paper is the first to conduct a quality assessment of scales used to measure CRF in people with cancer. Four scales meeting the most quality assessment criteria, within a cancer population, two unidimensional (BFI and FACT-F) and two multidimensional (MFI-20 and PFS- R). Consideration should be given when choosing an appropriate scale for research or clinical propose, as each scale measures different dimensions or aspects of CRF. The least likely criteria to be assessed included test-retest reliability, agreement, responsiveness, interpretation, and floor and ceiling effects. Given the importance of linking conceptional and operationalization of fatigue in people with cancer, recommendations to move forward with a universally accepted definition of fatigue would further help to advance healthcare science and clinical practice.
Supplemental Material
Download MS Word (154.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Campos MPO, Hassan BJ, Riechelmann R, et al. Cancer-related fatigue: a practical review. Ann Oncol. 2011;22:1273–1279.
- de Raaf PJ, de Klerk C, Timman R, et al. Differences in fatigue experiences among patients with advanced cancer, cancer survivors, and the general population. J Pain Symptom Manage. 2012;44:823–830.
- Rautalin M, Färkkilä N, Sintonen H, et al. Health-related quality of life in different states of breast cancer - comparing different instruments. Acta Oncol. 2018;57:622–628.
- Berger AM, Mitchell SA, Jacobsen PB, et al. Screening, evaluation, and management of cancer-related fatigue: ready for implementation to practice? Cancer-related fatigue. Cancer J Clin. 2015;65:190–211.
- Hoffman KE, McCarthy EP, Recklitis CJ, et al. Psychological distress in long-term survivors of adult-onset cancer: results from a national survey. Arch Intern Med. 2009;169:1274–1281.
- Güleser GN, Taşci S, Kaplan B. The experience of symptoms and information needs of cancer patients undergoing radiotherapy. J Canc Educ. 2012;27:46–53.
- Manir KS, Bhadra K, Kumar G, et al. Fatigue in breast cancer patients on adjuvant treatment: course and prevalence. Indian J Palliat Care. 2012;18:109.
- Weis J. Cancer-related fatigue: prevalence, assessment and treatment strategies. Expert Rev Pharmacoecon Outcomes Res. 2011;11:441–446.
- Wang XS, Woodruff JF. Cancer-related and treatment-related fatigue. Gynecol Oncol. 2015;136:446–452.
- Berger AM, Mooney K, Alvarez-Perez A, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Cancer-Related Fatigue, Version 1.2019 [Internet]. National Comprehensive Cancer Network; 2019 [cited 2019 Jun 4]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf.
- Radbruch L, Strasser F, Elsner F, et al. Fatigue in palliative care patients - an EAPC approach. Palliat Med. 2008;22:13–32.
- Mark W, Toulopoulou T. Psychometric properties of “community assessment of psychic experiences”: review and meta-analyses. Schizophr Bull. 2016;42:34–44.
- Vitoratou S, Pickles A. A note on contemporary psychometrics. J Ment Health. 2017;26:486–488.
- Huang F-F, Yang Q, Wang A, et al. Psychometric properties and performance of existing self-efficacy instruments in cancer populations: a systematic review. Health Qual Life Outcomes. 2018;16:241.
- Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF). Ann Oncol. 2009;20:17–25.
- Mota D, Pimenta C. Self-report instruments for fatigue assessment: a systematic review. Res Theory Nurs Pract. 2006;20:49–78.
- Seyidova-Khoshknabi D, Davis MP, Walsh D. Review article: a systematic review of cancer-related fatigue measurement questionnaires. Am J Hosp Palliat Care. 2011;28:119–129.
- Whitehead L. The measurement of fatigue in chronic illness: a systematic review of unidimensional and multidimensional fatigue measures. J Pain Symptom Manage. 2009;37:107–128.
- Terwee CB, Bot SDM, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34–42.
- Gough D, Oliver S, Thomas J. An introduction to systematic reviews. California, USA: SAGE; 2017.
- Eden MM, Kunkel K. Psychometric properties of the Modified Brief Fatigue Inventory and FACIT-Fatigue in individuals with cancer of the head and neck. Rehabil Oncol. 2016;34:97–103.
- Meek PM, Nail LM, Barsevick A, et al. Psychometric testing of fatigue instruments for use with cancer patients. Nurs Res. 2000;49:181–190.
- Shun S-C, Beck SL, Pett MA, et al. Assessing responsiveness of cancer-related fatigue instruments: distribution-based and individual anchor-based methods. Oncologist. 2007;12:495–504.
- Winstead-Fry P. Psychometric assessment of four fatigue scales with a sample of rural cancer patients. J Nurs Meas. 1998;6:111–122.
- Shun S-C, Beck SL, Pett MA, et al. Psychometric testing of three Chinese fatigue instruments in Taiwan. J Pain Symptom Manage. 2006;32:155–167.
- Ishikawa NM, Thuler LCS, Giglio AG, et al. Reproducibility of Functional Assessment of Cancer Therapy Fatigue (FACT-F) questionnaire for cancer patients. Appl Cancer Res. 2008;28:55–61.
- Jakobsson S, Taft C, Östlund U, et al. Performance of the Swedish version of the Revised Piper Fatigue Scale. Eur J Oncol Nurs. 2013;17:808–813.
- Stone P, Richards M, A'Hern R, et al. A study to investigate the prevalence, severity and correlates of fatigue among patients with cancer in comparison with a control group of volunteers without cancer. Ann Oncol. 2000;11:561–567.
- Schwartz AL, Meek PM, Nail LM, et al. Measurement of fatigue determining minimally important clinical differences. J Clin Epidemiol. 2002;55:239–244.
- Annunziata MA, Muzzatti B, Mella S, et al. The Revised Piper Fatigue Scale (PFS-R) for Italian cancer patients: a validation study. Tumori J. 2010;96:276–281.
- Lee EH. Construct validity of the revised Piper Fatigue Scale in Korean women with breast cancer. J Korean Acad Nurs. 1999;29:485–493.
- Lerdal A, Kottorp A, Gay C, et al. A Rasch analysis of assessments of morning and evening fatigue in oncology patients using the Lee Fatigue Scale. J Pain Symptom Manage. 2016;51:1002–1012.
- Mystakidou K, Tsilika E, Parpa E, et al. Psychometric properties of the brief fatigue inventory in Greek patients with advanced cancer. J Pain Symptom Manage. 2008;36:367–373.
- Piper BF, Dibble SL, Dodd MJ, et al. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25:677–684.
- So WKW, Dodgson J, Tai J. Fatigue and quality of life among Chinese patients with hematologic malignancy after bone marrow transplantation. Cancer Nurs. 2003;26:211–219.
- Åhsberg E, Fürst CJ. Dimensions of fatigue during radiotherapy–an application of the Swedish Occupational Fatigue Inventory (SOFI) on cancer patients. Acta Oncol Stockh Swed. 2001;40:37–43.
- Yun YH, Wang XS, Lee JS, et al. Validation study of the Korean version of the Brief Fatigue Inventory. J Pain Symptom Manage. 2005;29:165–172.
- Davis MP, Khoshknabi D, Walsh D, et al. Four-item fatigue screen: replacing the brief fatigue index. Am J Hosp Palliat Care. 2013;30:652–656.
- Mendoza TR, Laudico AV, Wang XS, et al. Assessment of fatigue in cancer patients and community dwellers: validation study of the Filipino version of the brief fatigue inventory. Oncology. 2010;79:112–117.
- Yoshimura A, Kobayashi K, Fumimoto H, et al. Cross-cultural validation of the Japanese functional assessment of cancer therapy-anemia (FACT-An). J Nippon Med Sch. 2004;71:314–322.
- Cantarero-Villanueva I, Fernández-Lao C, Díaz-Rodríguez L, et al. The piper fatigue scale-revised: translation and psychometric evaluation in Spanish-speaking breast cancer survivors. Qual Life Res. 2014;23:271–276.
- Wu H-S, McSweeney M. Assessing fatigue in persons with cancer: an instrument development and testing study. Cancer. 2004;101:1685–1695.
- Catania G, Bell C, Ottonelli S, et al. Cancer-related fatigue in Italian cancer patients: validation of the Italian version of the Brief Fatigue Inventory (BFI). Support Care Cancer. 2013;21:413–419.
- Radbruch L, Sabatowski R, Elsner F, et al. Validation of the German version of the brief fatigue inventory. J Pain Symptom Manage. 2003;25:449–458.
- Okuyama T, Wang XS, Akechi T, et al. Validation study of the Japanese version of the brief fatigue inventory. J Pain Symptom Manage. 2003;25:106–117.
- Yellen S, Cella D, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the functional assessment of cancer therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74.
- Dagnelie PC, Pijls-johannesma MCG, Pijpe A, et al. Psychometric properties of the revised Piper Fatigue Scale in Dutch cancer patients were satisfactory. J Clin Epidemiol. 2006;59:642–649.
- Giacalone A, Polesel J, De Paoli A, et al. Assessing cancer-related fatigue: the psychometric properties of the Revised Piper Fatigue Scale in Italian cancer inpatients. Support Care Cancer. 2010;18:1191–1197.
- Charalambous A, Kaite C, Constantinou M, et al. Translation and validation of the Cancer-Related Fatigue Scale in Greek in a sample of patients with advanced prostate cancer. BMJ Open. 2016;6:e011798.
- Hirai K, Kanda K, Takagai J, et al. Development of the Hirai Cancer Fatigue Scale: testing its reliability and validity. Eur J Oncol Nurs. 2015;19:427–432.
- Wu H-S, Wyrwich KW, McSweeney M. Assessing fatigue in persons with cancer: further validation of the Wu Cancer Fatigue Scale. J Pain Symptom Manage. 2006;32:255–265.
- Şahin S, Huri M, Aran OT, et al. Cross-cultural adaptation, reliability, and validity of the Turkish version of the Cancer Fatigue Scale in patients with breast cancer. Turk J Med Sci. 2018;48:124–130.
- Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196.
- Stone P, Hardy J, Broadley K, et al. Fatigue in advanced cancer: a prospective controlled cross-sectional study. Br J Cancer. 1999;79:1479–1486.
- Buss T, Kruk A, Wiśniewski P, et al. Psychometric properties of the polish version of the multidimensional fatigue inventory-20 in cancer patients. J Pain Symptom Manage. 2014;48:730–737.
- Fillion L, Gélinas C, Simard S, et al. Validation evidence for the French Canadian adaptation of the Multidimensional Fatigue Inventory as a measure of cancer-related fatigue. Cancer Nurs. 2003;26:143–154.
- Tian J, Hong JS. Validation of the Chinese version of Multidimensional Fatigue Inventory-20 in Chinese patients with cancer. Support Care Cancer. 2012;20:2379–2383.
- Okuyama T, Akechi T, Kugaya A, et al. Development and validation of the cancer fatigue scale: a brief, three-dimensional, self-rating scale for assessment of fatigue in cancer patients. J Pain Symptom Manage. 2000;19:5–14.
- Holley SK. Evaluating patient distress from cancer-related fatigue: an instrument development study. Oncol Nurs Forum. 2000;27:1425–1431.
- Chan A, Lew C, Wang XJ, et al. Psychometric properties and measurement equivalence of the Multidimensional Fatigue Syndrome Inventory- Short Form (MFSI-SF) amongst breast cancer and lymphoma patients in Singapore. Health Qual Life Outcomes. 2018;16:20. Available from: https://hqlo.biomedcentral.com/articles/10.1186/s12955-018-0846-6.
- Dapueto JJ, del Carmen Abreu M, Francolino C, et al. Psychometric assessment of the MSAS-SF and the FACIT-FATIGUE SCALE in Spanish-speaking patients with cancer in Uruguay. J Pain Symptom Manage. 2014;47:936–945.
- Baró E, Carulla J, Cassinello J, et al. Development of a new questionnaire to assess patient perceptions of cancer-related fatigue: item generation and item reduction. Value Health. 2009;12:130–138.
- Lai J, Cella D, Dineen K, et al. An item bank was created to improve the measurement of cancer-related fatigue. J Clin Epidemiol. 2005;58:190–197.
- Lin CC, Chang AP, Chen ML, et al. Validation of the Taiwanese version of the brief fatigue inventory. J Pain Symptom Manage. 2006;32:52–59.
- Baró E, Carulla J, Cassinello J, et al. Psychometric properties of the perform questionnaire: a brief scale for assessing patient perceptions of fatigue in cancer. Support Care Cancer. 2011;19:657–666.
- Wang XS, Hao X-S, Wang Y, et al. Validation study of the Chinese version of the Brief Fatigue Inventory (BFI-C). J Pain Symptom Manage. 2004;27:322–332.
- Paramita N, Nusdwinuringtyas N, Annisa Nuhonni S, et al. Validity and reliability of brief fatigue inventory (BFI)-Indonesian version in cancer patients. J Pain Symptom Manage. 2016;52:744–751.
- Aynehchi BB, Obourn C, Sundaram K, et al. Validation of the modified brief fatigue inventory in head and neck cancer patients. Otolaryngol Head Neck Surg. 2013;148:69–74.
- FACIT. FACIT Trans Home [Internet]. 2018 [cited 2018 May 14]. Available from: http://www.facit.org/TransHome.
- Van Belle S, Paridaens R, Evers G, et al. Comparison of proposed diagnostic criteria with FACT-F and VAS for cancer-related fatigue: proposal for use as a screening tool. Support Care Cancer. 2005;13:246–254.
- Meysami A, Yari H, Haghighat S, et al. The functional assessment of chronic illness Therapy - fatigue (FACIT-Fatigue Subscale): validity and reliability of the Iranian version. Oncol Res Treat. 2017;40:789–793.
- Ishikawa NM, Thuler LCS, Giglio AG, et al. Validation of the Portuguese version of Functional Assessment of Cancer Therapy-Fatigue (FACT-F) in Brazilian cancer patients. Support Care Cancer. 2010;18:481–490.
- Michielsen HJ, De Vries J, Van Heck GL, et al. Examination of the dimensionality of fatigue: the construction of the Fatigue Assessment Scale (FAS). Eur J Psychol Assess. 2004;20:39.
- Michielsen HJ, De Vries J, Van Heck GL. Psychometric qualities of a brief self-rated fatigue measure: the Fatigue Assessment Scale. J Psychosom Res. 2003;54:345–352.
- De Vries J, Van der Steeg AF, Roukema JA. Psychometric properties of the Fatigue Assessment Scale (FAS) in women with breast problems. Int J Clin Health Psychol. 2010;10:125–139.
- Krupp LB, LaRocca NG, Muir NJ, et al. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123.
- Smets EMA, Garssen B, Bonke B, et al. The multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–325.
- Smets E, Garssen B, Cull A, et al. Application of the multidimensional fatigue inventory (MFI-20) in cancer patients receiving radiotherapy. Br J Cancer. 1996;73:241–245.
- Baptista RLR, Biasoli I, Scheliga A, et al. Psychometric properties of the multidimensional fatigue inventory in Brazilian Hodgkin’s lymphoma survivors. J Pain Symptom Manage. 2012;44:908–915.
- Chandel P, Sultan A, Khan KA, et al. Validation of the Hindi version of the Multidimensional Fatigue Inventory-20 (MFI-20) in Indian cancer patients. Support Care Cancer. 2015;23:2957–2964.
- Hagelin CL, Wengström Y, Runesdotter S, et al. The psychometric properties of the Swedish Multidimensional Fatigue Inventory MFI-20 in four different populations. Acta Oncol Stockh Swed. 2007;46:97–104.
- Stein KD, Martin SC, Hann DM, et al. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6:143–152.
- Stein KD, Jacobsen PB, Blanchard CM, et al. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage. 2004;27:14–23.
- Pien LC, Chu H, Chen WC, et al. Reliability and validity of a Chinese version of the Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF-C). J Clin Nurs. 2011;20:2224–2232.
- Schwartz AL. The Schwartz Cancer Fatigue Scale: testing reliability and validity. Oncol Nurs Forum. 1998;25:711–717.
- Schwartz A, Meek P. Additional construct validity of the Schwartz Cancer Fatigue Scale. J Nurs Meas. 1999;7:35–45.
- Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7:301–310.
- Hann DM, Denniston MM, Baker F. Measurement of fatigue in cancer patients: further validation of the Fatigue Symptom Inventory. Qual Life Res. 2000;9:847–854.
- Piper BF. Piper fatigue scale available for clinical testing. Oncol Nurs Forum. 1990;17:661–662.
- Lundgren-Nilsson Å, Dencker A, Jakobsson S, et al. Construct validity of the Swedish version of the revised piper Fatigue Scale in an oncology sample—a Rasch analysis. Value Health. 2014;17:360–363.
- Mota D, Pimenta CAM, Piper BF. Fatigue in Brazilian cancer patients, caregivers, and nursing students: a psychometric validation study of the Piper Fatigue Scale-Revised. Support Care Cancer. 2009;17:645–652.
- Reeve BB, Stover AM, Alfano CM, et al. The Piper Fatigue Scale-12 (PFS-12): psychometric findings and item reduction in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2012;136:9–20.
- Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991;36:291–298.
- Belza BL. Comparison of self-reported fatigue in rheumatoid arthritis and controls. J Rheumatol. 1995;22:639–643.
- Okuyama T, Akechi T, Kugaya A, et al. Factors correlated with fatigue in disease-free breast cancer patients: application of the Cancer Fatigue Scale. Support Care Cancer. 2000;8:215–222.
- Kröz M, Zerm R, Reif M, et al. Validation of the German version of the Cancer Fatigue Scale (CFS-D). Eur J Cancer Care (Engl). 2008;17:33–41.
- Cella D, Viswanathan HN, Hays RD, et al. Development of a Fatigue and Functional Impact Scale in anemic cancer patients receiving chemotherapy. Cancer. 2008;113:1480–1488.
- Chou H-L, Hsieh P-C, Yao C-T, et al. Validity and reliability of the Taiwanese version of the General Fatigue Scale in cancer patients. Cancer Nurs. 2016;39:495.
- Agasi-Idenburg C, Velthuis M, Wittink H. Quality criteria and user-friendliness in self-reported questionnaires on cancer-related fatigue: a review. J Clin Epidemiol. 2010;63:705–711.
- Terwee CB, Prinsen CAC, Chiarotto A, et al. COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a Delphi study. Qual Life Res. 2018;27:1159–1170.