Abstract
Introduction: We hypothesized that gross tumor volume (GTV) of primary tumor (GTVT) and nodal volumes (GTVN) were predictors of first failure site in non-small cell lung cancer (NSCLC). We aimed at also comparing the prognostic model’s complexity to its ability to generate absolute risk predictions with emphasis on variables available at the time of diagnosis.
Materials and methods: Three hundred and forty-two patients treated with definitive chemoradiotherapy (CRT) for adenocarcinoma (AC) or squamous cell carcinoma (SCC) in 2009–2017 were analyzed. Clinical data, standardized uptake values on FDG-PET/CT, GTVT and GTVN were analyzed using multivariate competing risk models.
Results: One hundred and thirty-seven patients had SCC. As first site of failure 49 had locoregional failure (LRF), 40 had distant metastasis (DM) and 24 died with no evidence of disease (NED). In 205 patients with AC, 34 had LRF, 118 had DM as first failure site and 17 died with NED. Performance status predicted LRF (p = .02) and UICC stage risk of DM (p = .05 for stage 3, p < .001 for stage 4). Adding histopathology changed predictions with much reduced risk of LRF in AC compared to SCC (HR = 0.5, 95% CI: (0.3–0.75), p = .001). Conversely, AC had a higher rate of DM than SCC (HR = 2.1, 95% CI: (1.5–3.0], p < .001). Addition of FDG metrics and tumor/nodal volume data predicted DM risk (p = .001), but with smaller impact on absolute risk compared to histopathology. Separation of GTV in nodal and tumor lesions did not improve risk predictions.
Conclusions: We quantified the effect of adding volumetric and quantitative imaging to competing risk models of first failure site, but did not find tumor volume components to be important. Histopathology remains the simplest and most important factor in prognosticating failure patterns in NSCLC.
Introduction
Clinical stage is an anatomy-based biomarker that has demonstrated extensive prognostic relevance and is used daily in clinical routine to guide treatment of non-small cell lung cancer (NSCLC). However, the clinical stage and TNM-systems focus on overall survival prognostication and do not address the question of the most probable site of recurrence. The question of most likely failure mode (locoregional failure (LRF), distant metastasis (DM) or competing risk of death without prior disease progression) is of particular relevance to radiotherapy for NSCLC where tumor control remains a substantial challenge and severe toxicity limits the possibility of treatment intensification [Citation1,Citation2]. A failure-type specific prognostic tool may be of relevance both in patient counseling, treatment decision support and not least in selecting patients for clinical trials of intensified treatment regimens.
FDG-PET/CT imaging techniques have become increasingly central in radiotherapy (RT) planning for patients diagnosed with NSCLC. In addition to biopsy results, imaging provides the possibility of assessing relevant targets for RT and enabling definition of these for treatment purposes [Citation3,Citation4]. However, imaging may be of value beyond tumor tissue morphology and it is envisioned that quantitative imaging as a biomarker can be used to improve risk-stratification and individualize patient treatment [Citation5–8].
In a recent study conducted at Rigshospitalet, Copenhagen University Hospital, [Citation7], a competing risk model was developed for NSCLC applying several variables available at time of treatment planning. Histopathological subtypes, adenocarcinoma (AC) or squamous cell carcinoma (SCC), proved to be convincing prognostic markers of first failure site (LRF or DM) after completion of definitive chemo/radiotherapy. The study did not, however, include the gross tumor volume of primary tumor (GTVT) and involved lymph nodes (GTVN) when predicting risk of LRF and DM. One may speculate that the separated tumor volumes as measures of nodal vs. primary tumor burden are stronger drivers than histophatology for first site of failure prognostication, or that the histopathological drive of failure site could be caused by underlying differences in lesion sizes.
The aim of the present study is, therefore, to explore the effect of GTVT and GTVN when assessing the risk of LRF and DM in NSCLC. We hypothesized that lesions of greater tumor-volume are at higher risk of LRF as first failure, whereas greater volume of malignant lymph nodes entails increased risk of DM as first site of relapse. To do so, we enhanced the previously published competing risk model [Citation7] with GTVT and GTVN and updated the patient population.
A second objective is to compare models of different complexity with focus on the applicability of such models for trial enrichment or risk-adapted inclusion in clinical trials. Here, an increased prognostic accuracy of a failure type prognostic model must be balanced against the availability of data at the point of patient inclusion; detailed delineations or FDG PET analysis as a prerequisite for trial inclusion may drive up cost, drive down accrual or have an adverse impact on the time from diagnosis to treatment start. Consequently, a statistically significant superior model in accuracy may not be the most suitable model in clinical practice.
Materials and methods
Data from consecutive patients diagnosed with inoperable, locally advanced NSCLC and treated at Rigshospitalet, Copenhagen University Hospital, were retrieved retrospectively from medical journals and archived scans from January 2009 to December 2017. A multidisciplinary tumor board determined the treatment of the patients based on clinical stage, lung function, and comorbidity. The patients received either definitive chemoradiotherapy (CRT) or radiotherapy (RT) alone. CRT followed either a sequential regimen prior to RT or a concomitant regimen with one cycle of induction chemotherapy. Three cycles of either Cisplatin/Vinorelbine or Carboplatin/Vinorelbine chemotherapy were administered for the concomitant treatment and three to six cycles for the sequential treatment. Patients were included in the present study if they were treated with surgery for a previous early-stage lung cancer but currently referred to CRT or RT. Reasons for treatment with CRT or RT after being treated with surgery could be a new primary tumor or relapse in a lymph node station. For staging, the seventh TNM classification from the Union for International Cancer Control was applied [Citation9]. Patients were followed post treatment according to departmental guidelines. As part of a new guideline, first follow up was 6 weeks after post RT as opposed to earlier guidelines with follow up after 3 months. As this was implemented during the studied patient population, first follow up varies between 6 weeks and 3 months. After first follow up, patients were followed every three months for two years, subsequently every six months for a total of five years. At every follow up, a CT scan and physical evaluation was performed. Relapses were diagnosed with a CT scan, if in doubt a biopsy, MR- or PET/CT-scans were performed for verification.
Contouring and radiotherapy
A planning FDG-PET/CT scan in the treatment position was recorded prior to RT. The CT had diagnostic quality and intravenous contrast injection. The PET/CT acquisition followed strict departmental procedures as earlier described [Citation10]. When assessing scans with multiple FDG-positive lesions, a maximum of two tumor sites (T-sites, e.g. primary tumor and satellite tumor) and three lymph node sites (N-sites) were contoured. In cases with more than five FDG-positive lesions, the five lesions with highest uptake and greatest diameter were selected. Contouring of the lesions was performed by drawing semi-automatically with a region of interest (ROI) applying a threshold of 50% of the maximum standardized uptake value (SUVmax) followed by manual adjustment. For each selected FDG-avid lesion, SUVmax, SUVpeak, SUVmean, and volume (in cm3) were calculated. In accordance with the PERCIST-criteria [Citation11], SUVpeak was defined as a sphere of 1 cm3 centering on the hottest point in the lesion. In cases where the metabolic tumor volume of the primary tumor site was inseparable from the metabolic tumor volume of an FDG-positive lymph node, the entire lesion was contoured as a T-site lesion. Lesions were contoured and analyzed on a Mirada XD workstation, version 1.1.0.31 (Mirada Medical, Oxford, UK). FDG-negative lesions were included in the analysis as zero uptake.
The GTVs for T-sites and N-sites were obtained from the corresponding CT planning scan. The final GTVs had been delineated, evaluated, and, if needed, revised by a team of specialists in lung cancer radiotherapy as part of the RT-planning. In the population of patients diagnosed and treated prior to February 2015 there was no separation of GTVT and GTVN in clinical routine. For cases treated prior to February 2015, retrospective separation of a joint GTV into GTVT and GTVN was performed by TVL under supervision of a specialist in lung cancer radiotherapy. Separation of the primary tumor volume and lymph node volume was central to our analysis. Although T-sites and N-sites are often closely related, GTV delineation encloses a narrow area including only the malignant sites as visualized on the planning CT, often allowing for separation of lesions in primary tumor and involved lymph nodes. If a lesion was inseparable in primary tumor and lymph, the patient was excluded. This was feasible due to a relatively low amount of patients with inseparable lesions on a GTV-level (see “Results”). Contouring and retrieval of GTV-delineations was performed in ARIA Oncology Information System, version 15.1.0.6 (Varian Medical Systems, Inc., Palo Alto, CA, USA). Patients without a GTVN were included in the multivariable analysis with zero volume for GTVN.
Patients were treated with RT administered in 2-Gy fractions, five fractions per week, to a total dose of 60–66 Gy. Treatment was predominantly planned with three-dimensional conformal therapy planning techniques until January 2014, and predominantly with volumetric modulated arc therapy thereafter. The mid ventilation phase of a four-dimensional CT-scan [Citation12] was available for all patients and used for planning. In cases with small tumor motion (<0.5 cm), however, the 3D PET/CT with contrast could be used for planning. Treatment position was verified by daily cone beam CT scans prior to each fraction.
This study was approved by the Danish Health and Medicines Authority (case no. 3-3013-2836/1). Danish law does not prescribe research ethics approvals for retrospective studies.
Statistics and data analyses
The starting date was defined as the date of histopathological verification of NSCLC. This was chosen, as biopsies were taken at the same point in the diagnostic process before any treatment was initiated. Date of conclusive determination of recurrence based on imaging (FDG-PET/CT/MR) or a biopsy was determined as first failure-date. Scans performed due to suspicion of recurrence without conclusion were ignored regardless of later disease progression to avoid backdating issues. Patients with no evidence of disease (NED) were censored at their last follow-up date in an oncologic setting.
First failure was separated into groups of LRF and DM. LRF was defined as failure-site(s) included in the planning target volume (PTV) of the RT planning, either in the primary tumor/satellite tumor (local failure) and/or involved lymph nodes (regional failure). Distant metastases were defined as failure sites outside the PTV. If LRF and DM occurred simultaneously at first sign of progression, the patient was coded with DM, as the sample size was deemed insufficient for a separate subgroup with simultaneous failures.
Wilcoxon–Mann–Whitney U tests were used to compare AC and SCC baseline clinical parameters of ordinal or continuous nature. Chi-square tests were applied to measure for associations between categorical variables (). Kaplan–Meier plots [Citation13] and univariate Cox regression were applied for analysis of overall survival (OS) and time to progression (TTP). IBM SPSS Statistics for Windows, version 22.0.0.0 (IBM Corp., Armonk, NY, USA) was used for these analyses.
Table 1. Patient and treatment characteristics by histopathologic subtype.
A statistical plan was defined prior to analysis. The covariables included in the multivariate analysis are shown in . These were preselected, focusing on a limited number of promising, clinical prognosticators when building the model without volumetric data. Compared to our previous study [Citation7], GTV was excluded in favor of separate GTVT and GTVN.
Table 2. Subdistribution of hazard ratios in competing risk models: locoregional failure or distant metastases as first site of failure.
Multivariate regression was carried out using the CSC (cause-specific Cox) function of the RiskRegression package, version 1.4.3 in R [Citation14].
Results
Of the 184 patients screened in the current study (patient population from March 2015 to December 2017), 106 were deemed potentially eligible for analysis. The eligibility criteria were retrievable medical journals, retrievable RT planning FDG-PET/CT scans, completed RT, histopathology of either AC or SCC and PET-positive lesions. Together with the patient population from the earlier study [Citation7], this constituted a population of 357 patients potentially eligible for analysis. The lesions of the patients were assessed and, if necessary, retrospectively contoured. Fifteen patients were found to have inseparable T-site and N-site lesions on a GTV-contouring level, thus deeming these patients ineligible for analysis based on GTVT and GTVN. Therefore, a final number of 342 patients were included in the study (see Supplementary Figure 1). See for patient characteristics. Seven patients lacked data on performance status. These were retrospectively assessed on basis of medical journal information.
Twenty-seven patients were diagnosed with stage IV disease. Among these, 17 patients had M1a disease. These lesions were possible to include in PTV and, therefore, received RT with curative intent. Ten patients had brain metastases at the time of diagnosis, these were either surgically resected or received stereotactic RT prior to curative-intent treatment of the lung lesions. Two of the patients presenting with M1b disease had metastases in the left breast region and adrenal gland. Simultaneously with RT for their lung cancer lesions, these lesions received up to 34 Gy and stereotactic RT, respectively.
One hundred and nine patients experienced LRF as first relapse, including 100 relapses in primary and/or satellite tumor (local failure), and 28 relapses in one or more involved lymph nodes (regional failure). Among the 158 patients coded with DM as first relapse, 29 patients relapsed in thoracic lymph nodes outside of the original PTV, while 51 patients had a new thoracic lesion outside of the PTV.
A total of 729 FDG-avid lesions were observed in the 342 patients at the point of treatment planning. Three hundred and sixty-four of these were T-sites (322 primary lesions and 42 satellite tumors), of which 101 failed locally (97 primary lesions and 4 satellite tumors), equaling a crude relapse rate of 27.7%. Among the 365 FDG-positive N-sites, 39 relapsed regionally, equaling a crude failure rate of 10.7%.
Median time from diagnosis to first failure was 14.1 months (95% CI: (12.7–15.6)), while the median time from first relapse to death was 8.7 months (95% CI: (6.9–10.6)). The median overall survival for the entire patient population was 26.8 months (95% CI: (23.6–30.1)). Median follow up time was 20.8 months (95% CI: (18.0–23.6)).
Competing risk analysis
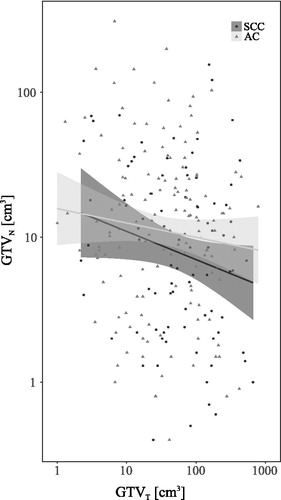
depicts GTVT and GTVN in all patients according to AC or SCC histopathology. We observed no association between histopathology and the relative tumor versus nodal tumor volume in the included patients.
Figure 1. Comparison of tumor volumes across histopathologic subtypes. GTVT: gross tumor volume of T-site; GTVN: gross tumor volume of N-site; SCC: squamous cell carcinoma; AC: adenocarcinoma.
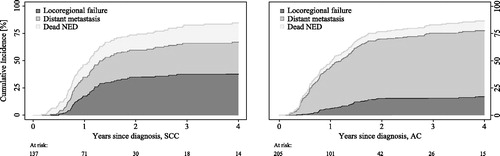
Univariate analyses showed that cumulative incidence rates of LRF and DM were associated with histopathology (). By Fine and Gray test, AC was shown to have a higher incidence rate of DM (p < .001), while SCC in comparison showed a greater incidence of LRF (p < .001). Patients with SCC had a higher incidence rate of death with NED than those with AC (p = .01).
Figure 2. First failure site by histopathologic subtype. Cumulative incidence rates of outcome variables by histopathology subtype. AC: adenocarcinoma; SCC: squamous cell carcinoma; DM: distant metastasis; NED: no evidence of disease.
Multivariate competing risk analysis failed to show a significant relationship between increasing GTVT/GTVN and first failure site. Histopathology, on the other hand, was the most powerful prognostic marker of first failure site. Patients with AC had half the risk of failing locoregionally compared to patients with SCC (HR = 0.5, 95% CI: (0.32–0.78), p = .002). However, AC had twice as high a risk of distant metastases as first failure compared to SCC (HR = 2.08, 95% CI: 1.44–3.01, p ≤ 0.001). SUVpeak for N-sites was able to predict DM in the expanded model (HR = 1.44, 95% CI: (1.15–1.79), p = .001); see .
Risk-prediction models were compared according to varying model complexity; see Supplementary Figure 2. The addition of histopathology to UICC stage and performance status had a substantial impact on the predicted risk profile. Absolute risk predictions of LRF differed 5–25%-points between models. Predicted risk of DM failure differed 0–42%-points.
Discussion
In this study, we expanded a previously published competing risk model for prediction of first failure site. By including separate volumes for tumor and nodal sites in the model, we hypothesized that increasing GTVT and GTVN would be predictive of LRF and DMs, respectively. A hypothesis that the histopathological drive of failure site was in fact caused by an underlying difference in lesion sizes (see ) could not be proven through competing risk analysis and we could not confirm our initial hypothesis that GTVT and GTVN would improve risk profiling. Our analyses of an expanded patient population demonstrated that histopathologic subtype continued to be the strongest predictor of first failure site in locally advanced non-small cell lung cancer, also in the presence of lesion volumetrics separated by lesion type. This is in accordance with previous findings and with others’ recent findings as well [Citation7,Citation15].
It should be noted that the current study included the previously published population and, therefore, should not be seen as an external validation of that study’s model. Nevertheless, the current study helps in identifying the most parsimonious and clinically relevant model for an ongoing effort to externally validate the present findings.
The incidence of dead with NED was significantly higher for patients with SCC. This suggests that these patients have a different risk profile, e.g. comorbidity, than do patients with AC. Age and performance status did not seem to increase the risk of this outcome, cf. the competing risk analysis.
An additional goal of this study was to compare the complexity of prognostic models to their applicability at the time of treatment decision or risk-adapted inclusion in clinical trials. When comparing a simplified model containing only data from the time of diagnosis to a full model including volumetric data, we found that the full model only alters absolute risk prediction modestly. This is a clinically important finding, as it informs the use of a simplified model for decision support on an individual level as for inclusion into future trials.
This is, to our knowledge, the first study emphasizing on comparing the complexity of a failure-prediction model to its ability to produce absolute risk predictions at the time of diagnosis. A number of studies have previously investigated the relevance of FDG-PET parameters for failure patterns [Citation16,Citation17], while others have focused on building prediction models for survival in patients with NSCLC [Citation18]. However, there continues to exist a need for further research in the field of prognostic tools for estimation of locoregional or distant recurrence after CRT for NSCLC [Citation19]. We have addressed this need; our goal being to build a tool for patients with locally advanced NSCLC that can help differentiate patients for trial inclusion and for treatment.
In conclusion, detailed knowledge of GTVT and GTVN did not improve the risk profiling of NSCLC patients. The strongest and most readily available predictor of failure type was tumor histopathology and it may be beneficial to separate AC and SCC in clinical research to better target the dominant risk for these patients in future trials. Knowledge of FDG uptake appears to allow further risk stratification but must be weighed against its availability at time of diagnosis.
Supplemental Material
Download MS Word (306.1 KB)Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial p. Lancet Oncol. 2015;16:187–199.
- Cannon DM, Mehta MP, Adkison JB, et al. Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol. 2013;31:4343–4348.
- Spratt DE, Diaz R, McElmurray J, et al. Impact of FDG PET/CT on delineation of the gross tumor volume for radiation planning in non-small-cell lung cancer. Clin Nucl Med. 2010;35:237–243.
- Konert T, Vogel W, MacManus MP, et al. PET/CT imaging for target volume delineation in curative intent radiotherapy of non-small cell lung cancer: IAEA consensus report 2014. Radiother Oncol. 2015;116:27–34.
- O'Connor JPB, Aboagye EO, Adams JE, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol. 2017;14:169–186.
- Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749–762.
- Nygård L, Vogelius IR, Fischer BM, et al. A competing risk model of first failure site after definitive chemoradiation therapy for locally advanced non-small cell lung cancer. J Thorac Oncol. 2018;13:559–567.
- Ohri N, Duan F, Snyder BS, et al. Pretreatment 18F-FDG PET textural features in locally advanced non-small cell lung cancer: secondary analysis of ACRIN 6668/RTOG 0235. J Nucl Med. 2016;57:842–848.
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–714.
- Nygård L, Vogelius IR, Fischer BM, et al. Early lesion-specific (18)F-FDG PET response to chemotherapy predicts time to lesion progression in locally advanced non-small cell lung cancer. Radiother Oncol. 2016;118:460–464.
- Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S–150S.
- Wolthaus JWH, Sonke J-J, van Herk M, et al. Comparison of different strategies to use four-dimensional computed tomography in treatment planning for lung cancer patients. Int J Radiat Oncol Biol Phys. 2008;70:1229–1238.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481.
- R: The R Project for Statistical Computing. 2008. [accessed 19/03/2019]. Available from: https://www.r-project.org/.
- Kim E, Wu H-G, Keam B, et al. Significance of 18 F-FDG PET parameters according to histologic subtype in the treatment outcome of stage III non small-cell lung cancer undergoing definitive concurrent chemoradiotherapy. Clin Lung Cancer. 2019;20:9–23.
- Kandi M, Hoffmann L, Sloth Moeller D, et al. Local failure after radical radiotherapy of non-small cell lung cancer in relation to the planning FDG-PET/CT. Acta Oncol. 2018;57:813–819.
- Ohri N, Bodner WR, Halmos B, et al. 18F-fluorodeoxyglucose/positron emission tomography predicts patterns of failure after definitive chemoradiation therapy for locally advanced non-small cell lung cancer. Int J Radiat Oncol. 2017;97:372–380.
- Jochems A, El-Naqa I, Kessler M, et al. A prediction model for early death in non-small cell lung cancer patients following curative-intent chemoradiotherapy. Acta Oncol. 2018;57:226–230.
- Walls GM, Hanna GG, Qi F, et al. Predicting outcomes from radical radiotherapy for non-small cell lung cancer: a systematic review of the existing literature. Front Oncol. 2018;8:433.
