Abstract
Introduction: Stereotactic radiotherapy (SBRT) is the treatment of choice for inoperable early stage non-small cell lung cancer (NSCLC). We report analyses of the influence of age on survival after SBRT.
Methods and material: From 2005 to 2017, 544 previously un-irradiated patients with early stage NSCLC had SBRT. The data were analyzed in four age groups: A: –69 (176 pts), B: 70–74 (115 pts), C: 75–79 (131 pts) and D: 80 years or older (122 pts). Two SBRT dose regimes were used: 45 Gy/3F (N = 103) and 66 Gy/3F (N = 441).
Results: All patients had a follow up (time to censoring, FU) of at least 16 months, the median FU being 48.0 months. The median age was 74.4 years. The overall survival (OS) was associated with age. The median OS was 50.7, 45.9, 45.4 and 33.0 months, and the 5-year OS was 45%, 32%, 33% and 18% in groups A, B, C and D, respectively. No difference was found between groups B and C, while OS in group A was significantly better than remaining groups, and the OS in group D significantly poorer. In multivariable analyses, OS was heavily influenced by age, Charlson’s comorbidity index (CCI) and performance status (PS). For lung cancer-specific survival (LCSS), only increasing tumor diameter and PS were associated with poor survival.
Conclusions: The OS was influenced by age, but the study suggests that a cut point of 75 year is inappropriate in evaluating the effect of old age on survival. Poor PS was associated with poor OS. CCI influenced OS, but not LCSS, which was only affected by PS and tumor size.
Introduction
Stereotactic radiotherapy (SBRT) is the treatment of choice for inoperable patients with peripheral located early stage non-small cell lung cancer (NSCLC) [Citation1–3]. The median age for the European population is increasing and thus older patients will increasingly apply for treatment [Citation4]. SBRT is a convenient treatment and even older patients with NSCLC in poor performance status (PS) can cooperate to the treatment because of the short treatment schedule. Although the survival for untreated patients is poor [Citation5], and the treatment of old patients presents challenges due to, e.g., comorbidity and impaired organ function, several series have demonstrated that old patients may benefit from SBRT as much as younger do [Citation6,Citation7]. However, the studies have most often analyzed the data dichotomized using different ages as the cut point, most often 75, with rather small numbers of older patients [Citation7]. Even though SBRT now is an accepted treatment modality, it is unknown if all patients have the same benefit of the treatment due to the lack of randomized studies. There is therefore a lack of knowledge about the benefit for old patients as well as which cut point is the most appropriate to use for analyzing the outcome of treatment in old patients.
The commonly used cut point of 75 years is appropriate for evaluation of the influence of age on survival after SBRT, and we here report a retrospective study an unselected, but homogenous, cohort of patients with peripheral lesions of NSCLC treated with SBRT analyzed in four age groups.
Methods and material
Patients
From August 2005 to December 2017, 544 inoperable patients with early NSCLC with one peripheral lesion less than or equal to 5 cm were treated with SBRT. Previous surgery for lung cancer was allowed in the study, but not previous radiotherapy for lung cancer. Patients with a history of SCLC, more than one target lesion, regional or distant metastases were excluded from the study. FDG–PET–CT scans were used as standard procedure for staging. There was no restriction for the inclusion in study on pulmonary function (PF), PS, comorbidity or age. The data cut point was 1 May 2019 at which time the vital status of all patients was known. Data on sex, age, smoking habit, body mass index (BMI), histology, previous thoracic surgery, PFT (pulmonary function test measured as forced expiratory volume in one second, FEV1), date of death and cause of death were obtained for analyses. Additional data on comorbidity were collected to create the Charlson comorbidity index (CCI) [Citation8]. Tumor diameter was obtained from the planning CT-scans.
Radiation technique
All patients were treated with SBRT as previously described [Citation9]. In summary, the prescribed dose was 45 Gy in three fractions or 66 Gy in three fractions. The gross tumor volume (GTV) was covered by 95% of the prescribed dose with no maximum dose to the GTV. The planning target volume (PTV) was covered by 66% of the prescribed dose. Prior to October 2008, the 45 Gy dose was used exclusively. After October 2008, 66 Gy was used preferentially, unless the patient had prior surgery, or if the tumor was abutting the thoracic wall. In either of these cases, 45 Gy was prescribed. Maximum duration of the treatment course was nine days. For all patients, 4D planning CT, daily 4D CBCT with soft tissue match and IMRT/VMAT treatment technique was used. Flattening filter free (FFF) radiation therapy was introduced for stereotactic treatments in 2013.
Statistical methods/analyses
The analysis was constructed as a descriptive observational study. Four cohorts were established using different age cut points for patients with NSCLC treated with SBRT. The age-groups analyzed were: A: younger than 69 years (176 patients), B: 70–74 years (115 patients), C: 75–79 years (128 patients) and D: 80 years or older (122 patients).
The primary endpoint was overall survival (OS). The secondary endpoint was lung cancer-specific survival (LCSS). The survival rates were calculated from the date of SBRT start. OS was defined as the time from start of SBRT to death of any cause including lung cancer. Lung cancers specific survival was determined as death in patients with known recurrent disease, and the survival time was censored at the time of death in other situations. Survival was calculated using the Kaplan–Meier method, and the log rank test was used for testing differences in survival rates. p Values less than .05 were considered statistically significant. Multivariable analyses used a proportional Cox regression mode including age groups, CCI, PS, BMI, FEV1, tumor diameter (in cm), sex, tumor dose, previous surgery, histology and smoking history. The proportional hazard assumption was evaluated graphical and with tests of residuals.
Results
The median age was 74.4 years (range: 47.6–94.4 years). For further information of baseline patient characteristics, see . All patients had a potential follow up from start of treatment to death or censoring of at least 16 months. The median follow time was 48.0 months. At data cut point, 305 patients had died. The cause of death was known for 95% of the patients (). Of the included patients, 39% died of lung cancer.
Table 1. Patient characteristics.
Table 2. Causes of death in patients with early-stage non-small lung cancer treated with SBRT.
The OS was 43.9 months. The OS was associated with age (). The OS in age-groups B and C was almost identical, while the OS in group A was better than in groups B + C combined and group D. Likewise, OS in group D was poorer than groups B + C combined and group A. The median OS in groups A, B, C and D was 50.7, 45.9, 45.4 and 33.0 months, the 3-year OS 64%, 52%, 57% and 43%, and the 5-year OS was 45%, 32%, 33% and 18%.
Figure 1. (a) Overall survival after SBRT in early stage NSCLC. (b) Lung cancer specific survival after SBRT in early stage NSCLC.
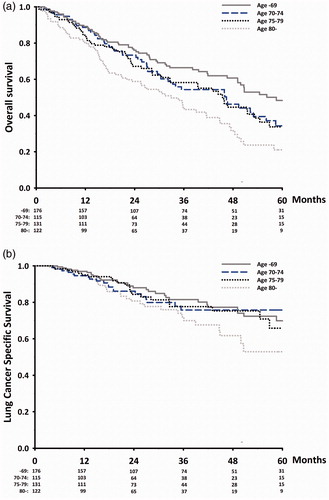
OS was influenced by PS. PS 0–1 was observed in 50% of the patients (). In these patients, the OS in group A was significantly better than each of the other age-groups, but the OS did not differ significantly in the other age groups. The median OS in groups A, B, C and D was 71.5, 45.9, 45.9 and 47.2 months.
For patients with PS 2–3, the survival was equal in the age groups A, B and C and statistically significantly lower in group D than in other age groups. The median OS in groups A, B, C and D was 38.1, 40.2, 32.6 and 20.8 months. The OS for the 13 patients aged ≥80 years with PS 3 was 16.6 months.
Since the univariate analyses of OS in age groups B and C was very similar, the age groups were collapsed into one age group leaving only three age groups for analyses: ≤69, 70–79 and ≥80 years. These age groups, CCI and PS (0–1/2–3) were the most significant factors associated with favorable OS (). The model also included male sex, BMI ( < 18.5 kg/m2), PFT (FEV1 < 50% of predicted) and the tumor diameter, while nonsignificant factors with p>.10 included non-adenocarcinoma histology, previous surgery, radiation dose and smoking habit.
Table 3. Overall survival: Cox proportional hazard analyses.
There was no statistically significant difference in LCSS between age groups A, B and C, while the LCSS among patients 80 years or older was statically significant poorer (p < .03, ). The 3-year LCSS was 88%, 76%, 78% and 72% in groups A, B, C and D, respectively, and the 5 year LCSS was 70%, 76%, 66% and 53%.
The 5 year LCSS was 67%, 76%, 63% and 51% in groups A, B, C and D, respectively, but the differences in LCSS were not statistically significant.
In Cox analyses of LCSS, PS and tumor diameter were significant factors while neither age, Charlson’s CI, nor any other factor was associated LCSS. HR for tumor size was HR 1.45 (95% CI 1.21: 1.74) per cm.
Discussion
The current dataset represents a large single-institutional cohort of patients with early stage NSCLC consecutively treated with SBRT at a University Hospital. The cohort was homogenous with respect to stage. All patients had only a single lesion, no previous radiotherapy for NSCLC, no regional lymph node metastases or distant metastases, and the histology of NSCLC was determined before treatment in 97% of the cases. The treatment was performed by just two dose levels utilizing the same technique including cone beam CT set up for each fraction and 4D-soft tissue guided planning and radiotherapy.
However, although the tumor-population was homogeneous, the patient-population in the study was not. The patients were not excluded on basis of comorbidity, PS, PF or age, and since the treatment is free of charge for all citizens in Denmark, the selection bias often introduced in retrospective studies due to the impact of socio-economic factor was kept to a minimum. Furthermore, since the exact date of death was known for all deceased patients and the cause of death for nearly all patients, this set of data offers a unique possibility to study the influence of age on the prognosis after treatment.
In published reports analyzing SBRT in early stage NSCLC, age is frequently dichotomized with varied cut points for being old. A recent review by Shinde et al. [Citation7] reported cut points in 17 studies: 65 years in two studies, 70 years in two studies, 75 years in six studies, 80 years in five studies, 85 years in one study and 90 years in one study. Besides, most studies are small, only four of the studies included more than 100 patients. The studies included both peripheral and central tumors, and used varied dose schedules and planning techniques. The follow-up varied between series. It is therefore difficult from the published data to infer to what extent age and other factors influence on the prognosis after SBRT for early stage NSCLC. The present data suggest that a cut point of 75 years is inappropriate when studying survival after SBRT since no difference was observed in OS between patients in the age groups 70–74 and 75–79. The observed differences in OS for patients younger than 70 and for octogenarians call for more detailed analyses of the impact of age data than just dichotomizing.
The 122 octogenarians had a significant poorer OS than younger age groups. The three-year OS was 45%, and that is in line with data from five series [Citation10–14] showing three year OS in the range of about 40–56%, with a median OS of approximately 43%. To our knowledge, no reliable five year OS data on octogenarians has been published. The five year OS in the present study was 18%, and even though this number seems low, these patients still benefit from SBRT. Very few patients survive with untreated local NSCLC as demonstrated by Jeppesen et al. [Citation5].
It was expected that age would influence OS since the expected life time of old patients is much less than the expected lifetime of young patients. Also PS influenced OS, but for patients in P 0–1 no differences between the three age groups of 70–74, 75–79 and ≥80 were found.
CCI ≥3 was associated with poor OS, which may seem an obvious finding. However, in a publication from the Danish Lung Cancer Registry, only a modest influence of CCI on OS was found for non-surgical patients with lung cancer where radiation was the initial treatment [Citation15]. However, the registry study included all lung cancer histology including SCLC, curative intended treatment and palliation, and all types of radiotherapy techniques mostly not SBRT. The registry data therefore cannot be compared with our very uniform selected data. With more efficient therapy, fewer patients will die of lung cancer and the proportion of death of other causes will rise. Deaths of other causes than lung cancer are likely to be associated with CCI. Of the same reasons it was expected that CCI that neither comorbidity nor age influenced LCSS.
In a recent study by Klement et al., it was not possible to predict early death within six months after SBRT by factor as age, sex, PS, operability, FEV1 and CCI [Citation16]. However, as in line with the present study, PS was the most important predictor of early death. A study by Cassidy et al. also demonstrated that for octogenarians PS predicted OS and that even for older patients with NSCLC that SBRT is safe and efficacious [Citation10].
In order to predict if an old patient with NSCLC will benefit for treatment with SBRT, a recent study by Maebayashi et al. screened a small number of patients with a geriatric tool Geriatric 8 (G8) [Citation17]. The patient was considered frail with a score ≤14. However, result of the study showed that patients with a G8 cut point of less than 12 might not benefit of SBRT due to death of inter-current diseases and that there was no association between age and early death soon after SBRT.
Since comorbidity influences OS, but not LCSS, it seems relevant to optimize the comorbid condition of the patients to improve survival. In a recent randomized pilot study, an attempt to improve outcome after SBRT for patients with early stage NSCLC, a comprehensive geriatric assessment (CGA) including interventions and follow-up at a Department of Geriatric Medicine was performed [Citation18]. The study found that more patients died within the first year after SBRT in the group without a CGA performed, but the result was not statistically significant. However, the study suggested that optimizing the patient comorbid conditions in older patients instead of only focusing on the cancer diagnosis may improve outcomes. This is in accordance with the recently published study by Mihai et al. who concluded that older adults can successfully complete SBRT, but might require increased supportive care for successful treatment delivery [Citation6].
The present study does not give the full picture of how age impacts the outcome after SBRT in lung cancer. However, it seems that withholding SBRT for old patients with early stage NSCLC is not justified, even if they are in poor PS. We have not analyzed toxicity after treatment, and we have only studied peripheral tumors. The impact of age on survival in central located tumors may be different, since the toxicity may be more severe as demonstrated by Timmerman et al. [Citation19]. It will also be interesting to analyze local tumor control in relation to age and comorbidity.
Conclusions
The survival after SBRT in old patients was excellent. The study suggests that a cut point of 75 years is inappropriate in evaluating the effect of old age on survival. For patients in favorable PS, the OS and LCSS for octogenarians were equal to patients aged 70–79. Poor PS was associated with poor OS. CCI influenced OS, but not LCSS, which was only affected by PS and tumor size.
Disclosure statement
No potential conflict of interest was reported by the authors..
References
- Guckenberger M, Andratschke N, Dieckmann K, et al. ESTRO ACROP consensus guideline on implementation and practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer. Radiother Oncol. 2017;124:11–17.
- Nyman J, Hallqvist A, Lund JA, et al. SPACE – a randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121:1–8.
- Ball D, Mai GT, Vinod S, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019;20:494–503.
- Kristiansen C, Schytte T, Hansen KH, et al. Trends in lung cancer in elderly in Denmark, 1980–2012. Acta Oncol. 2016;55 Suppl. 1:46–51.
- Jeppesen SS, Hansen NCG, Schytte T, et al. Survival of localized NSCLC patients without active treatment or treated with SBRT. Acta Oncol. 2018;57:219–225.
- Mihai A, Milano MT, Santos A, et al. Treatment completion, treatment compliance and outcomes of old and very old patients treated by dose adapted stereotactic ablative radiotherapy (SABR) for T1-T3N0M0 non-small cell lung cancer. J Geriatr Oncol. 2019;10:442–448.
- Shinde A, Li R, Kim J, et al. Stereotactic body radiation therapy (SBRT) for early-stage lung cancer in the elderly. Semin Oncol. 2018;45:210–219.
- Jorgensen TL, Hallas J, Friis S, et al. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer. 2012;106:1353–1360.
- Jeppesen SS, Schytte T, Jensen HR, et al. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: an updated retrospective study on local failure and survival rates. Acta Oncol. 2013;52:1552–1558.
- Cassidy RJ, Patel PR, Zhang X, et al. Stereotactic body radiotherapy for early-stage non-small-cell lung cancer in patients 80 years and older: a multi-center analysis. Clin Lung Cancer. 2017;18:551–558.
- Kreinbrink P, Blumenfeld P, Tolekidis G, et al. Lung stereotactic body radiation therapy (SBRT) for early-stage non-small cell lung cancer in the very elderly (≥80 years old): extremely safe and effective. J Geriatr Oncol. 2017;8:351–355.
- Sandhu AP, Lau SK, Rahn D, et al. Stereotactic body radiation therapy in octogenarians with stage I lung cancer. Clin Lung Cancer. 2014;15:131–135.
- Takeda A, Sanuki N, Eriguchi T, et al. Stereotactic ablative body radiation therapy for octogenarians with non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;86:257–263.
- van der Voort van Zyp N, van der Holt B, Van Klaveren RJ, et al. Stereotactic body radiotherapy using real-time tumor tracking in octogenarians with non-small cell lung cancer. Lung Cancer. 2010;69:296–301.
- Mellemgaard A, Lüchtenborg M, Iachina M, et al. Role of comorbidity on survival after radiotherapy and chemotherapy for nonsurgically treated lung cancer. J Thorac Oncol. 2015;10:272–279.
- Klement RJ, Belderbos J, Grills I, et al. Prediction of early death in patients with early-stage NSCLC—can we select patients without a potential benefit of SBRT as a curative treatment approach? J Thorac Oncol. 2016;11:1132–1139.
- Maebayashi T, Ishibashi N, Aizawa T, et al. Significance of stereotactic body radiotherapy in older patients with early stage non-small cell lung cancer. J Geriatr Oncol. 2018;9:594–599.
- Jeppesen SS, Matzen LE, Brink C, et al. Impact of comprehensive geriatric assessment on quality of life, overall survival, and unplanned admission in patients with non-small cell lung cancer treated with stereotactic body radiotherapy. J Geriatr Oncol. 2018;9:575–582.
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839.
