Abstract
Purpose: To determine if anal cancer patients with HPV positive disease have different overall survival (OS) compared to those with HPV negative disease, and to elucidate differences in the association between radiation dose and OS.
Patients and methods: We utilized the National Cancer Database (NCDB) registry to identify a cohort of non-metastatic anal cancer patients treated with curative intent between 2008 and 2014. Propensity score matching was used to account for potential selection bias between patients with HPV positive and negative disease. Multivariable Cox regression was used to determine the association between HPV status and OS. Kaplan–Meier methods were used to compare actuarial survival estimates.
Results: We identified 5927 patients with tumor HPV status for this analysis, 3523 (59.4%) had HPV positive disease and 2404 (40.6%) had HPV negative disease. Propensity-matched analysis demonstrated that patients with HPV positive locally advanced (T3–4 or node positive) anal cancer had better OS (HR = 0.81 (95%CI: 0.68–0.96), p=.018). For patients with early stage disease (T1–2 and node negative) there was no difference in OS (HR = 1.11 (95%CI: 0.86–1.43), p=.43). In the unmatched cohort, we found a significant improvement in OS with increasing radiation dose only for patients with locally advanced, HPV negative disease (p<.001). In those patients, significant improvement in OS compared to the group receiving 30–45 Gy was seen for increasing doses up to 55–60 Gy, but not beyond 60 Gy.
Conclusion: We found HPV to be a significant prognostic marker in anal tumors, especially for locally advanced disease. We further found that higher radiation dose up to 55–60 Gy was associated with better OS, but only for patients with locally advanced, HPV negative disease.
Introduction
Anal cancer accounts for an estimated 8500 new cases in 2018 in the USA and is considered a rare type of cancer [Citation1]. While concurrent chemotherapy and radiation therapy are the standard of care treatments for non-metastatic invasive anal cancer resulting in high cure rates, these treatments are also associated with significant acute and chronic side effects [Citation2–4]. Strategies that allow treatment de-intensification for the favorable subtypes as well as treatment intensification for the unfavorable subtypes are clearly needed.
The optimal radiation dose for anal cancer is not currently known. The initial Nigro experience delivered a total dose of only 30 Gy which resulted in a complete response in 24 out of 28 patients [Citation5]. Since that initial experience, most anal cancer clinical trials have used radiation doses ranging from 45 Gy up to 70 Gy. The single arm RTOG 92-08 could not prove a benefit to increasing the dose beyond 50 Gy, especially if this is delivered with a 2-week treatment break [Citation6]. Most recently, the Accord 03 trial tested a boost dose of 15 Gy compared to 20–25 Gy after initial 45 Gy. While there was a small increase in colostomy-free survival and local control with high-dose RT, this did not reach statistical significance (p = .067) and the study could not demonstrate a benefit for escalating dose beyond 60 Gy [Citation7]. It has also been shown that the dose-response of anal cancer tumors is considerably different depending on the size, as small tumors may in fact have a very shallow dose-response, with potential for dose de-escalation [Citation8].
Currently, there is an ongoing clinical trial (PLATO – PersonaLising Anal cancer radioTherapy dOse) testing whether reduced dose radiotherapy (41.4 Gy in 23 fractions vs. 50.4 Gy in 28 fractions) can be used for T1–T2N0 with tumors less than 4 cm and whether dose-escalated RT (58.8 Gy and 61.6 Gy, both in 28 fractions compared to 53.2 Gy in 28 fractions) can improve outcomes for T3–4 and node positive disease. Until results of prospective trials are available, current radiation dose recommendations continue to range widely from 45 Gy to 59 Gy or higher.
Anal cancer is associated with human papillomavirus infection, especially with high-risk HPV subtypes (HPV-16 and HPV-18), which are thought to be present in 68–97% of invasive anal cancer specimens [Citation9–12]. Small retrospective series have shown that HPV-positive anal cancers have better prognosis than those with HPV-negative anal cancers [Citation10,Citation12,Citation13]. Since HPV-associated oropharyngeal cancers have been shown to have improved survival as well as enhanced radiation sensitivity [Citation14,Citation15], we hypothesized that HPV associated anal cancer may have improved overall survival (OS) and potentially enhanced radiation sensitivity as well. We performed an analysis using the National Cancer Database (NCDB) registry to determine whether HPV status was associated with better OS and its implications on radiation dose.
Patients and methods
The NCDB is a joint project from the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society (ACS). Data are collected from more than 1500 participating centers and represent more than 70% of newly diagnosed cancer cases in the USA. The de-identified data within the NCDB come from CoC-participating hospitals; the data have not been verified and participating hospitals are not responsible for the validity of the data analysis or the conclusions derived by the authors. The data in the NCDB include patient demographics, cancer staging, comorbidities, information concerning therapies delivered during the first course of treatment, as well as OS.
The NCDB was used to identify patients with newly diagnosed, non-metastatic anal cancer between 2008 and 2014. Using 2008 as the starting point was chosen as the NCDB contains data on HPV for anal cancer patients from 2008 and onward. Patients with primary squamous cell carcinoma of the anus were included in the analysis. Staging was performed per institutional standards according to AJCC 7th edition. Patients were excluded if they met the following criteria: received no treatment or active surveillance, received palliative care, did not have available follow-up for OS, had T0 disease or had metastatic disease. Patients were subsequently stratified for analysis according to whether they had HPV positive or HPV negative disease based on pathologic examination of tumor specimens.
Participating centers were instructed to record the results of any HPV testing performed on pathologic specimens. This specimen could be the primary tumor or a metastatic site, including lymph nodes. They were further instructed not to record the results of HPV testing based on blood tests or serology.
The main hypothesis of this study was whether anal cancer patients with HPV positive disease had similar OS to patients with HPV negative disease. The secondary aim was to assess if there was any significant association between increasing radiation dose and OS, depending on HPV status and disease stage.
To adjust for the nonrandom distribution of potentially confounding variables that may have been influenced by patient and/or disease-related characteristics, we analyzed the association between HPV status and OS using a propensity score-matching strategy. Propensity scores were calculated using multivariable logistic regression with HPV status as the outcome variable and matched on age, sex, race, ethnicity, Charlson-Deyo co-morbidity score, T and N stage, systemic therapy, surgery of the primary site, type of facility and median income. A 1:1 propensity match was done to match patients with HPV positive disease to those with HPV negative disease using the psmatch2 function in STATA v.14 (StataCorp, College Station, TX, USA), not allowing replacement and using a caliper width equal to 0.005.
Baseline and treatment characteristics for the entire cohort, as well as for the propensity-matched cohort, were compared using χ2 tests for categorical variables and two-sided t-tests or the non-parametric equivalent for continuous variables.
To determine the unbiased association between HPV status and OS, we performed multivariable Cox proportional hazards regression modeling using the propensity-matched cohort. The proportional hazards assumption was assessed by formal test and visual inspection of the Schoenfeld residuals. Patient variables included in the survival analysis were HPV status, age, sex, race, ethnicity, Charlson-Deyo co-morbidity score, T and N stage, systemic therapy, surgery of the primary site, type of facility and median income. Kaplan–Meier survival curves for OS were generated for patients with HPV positive and HPV negative disease, stratified by early (T1–2 node negative) or locally advanced (T3–4 or node positive) disease stage. A two-sided p-value of <.05 was considered statistically significant.
For the association between total radiation dose and OS, the unmatched cohort was used and the same multivariable Cox regression model as derived above was used to determine the independent association between OS and increasing radiation dose from 30–44.9 Gy, 45–49.9 Gy, 50–54.9 Gy, 55–59.9 Gy and ≥60 Gy.
Results
We identified patients with cT1–T4, N0–N3, M0 anal cancer for this analysis, of which 5927 patients had information on HPV status from tumor specimens, with patient selection illustrated in Supplementary Figure S1. There were 5680 patients with information stating that HPV testing was not ordered or performed, and their baseline characteristics were compared with those who had HPV tested, as presented in . The distribution of HPV risk and type is presented in Supplementary Table S1, where 3523 (59.4%) patients had HPV positive disease of some type and 2404 (40.6%) had HPV negative disease. For those with HPV positive disease, HPV-16 was the most common type present in 76.0% of those with known HPV subtype, which for low-risk types was 13.3% and for high-risk types other than HPV-16 was 10.6%. There were 1661 HPV positive patients for whom the risk and subtype was not specified. Patients who were tested for HPV were younger and had a larger proportion of male patients than those who were not tested (p < .001). Patients tested for HPV also had a slightly larger proportion of patients in higher income quartiles (p = .004) and slightly more patients treated at an academic/research program (p = .017). About 53% of patients did not undergo any surgical procedure to the primary tumor site. For those who were listed as having surgery, the most common procedure was an excisional biopsy (49%), whereas the rest received either local excision (25%), abdominal perineal resection (15%), polypectomy (5%), local tumor destruction/ablation (4%) or surgery without further specification (2%).
Table 1. Patient characteristics in the unmatched cohort.
The 3- and 5-year OS rates for all patients were 78.1% and 69.9%, respectively, with a median follow-up of 37.8 months. The baseline patient and treatment characteristics are shown in for all patients and stratified by HPV status. Patients with HPV positive disease were younger (54.9 years vs. 60.8 years, p < .001), more likely to be male (46.0% vs. 35.0%, p < .001), have poor performance status (Charlson-Deyo score ≥2 in 12.0% vs. 6.4%, p < .001), have node negative disease (76.2% vs. 72.0%, p < .001) and not receive any systemic therapy (37.0% vs. 25.6%, p < .001), compared to patients with HPV negative disease. The total radiation therapy dose was similar between those with HPV positive disease and those with HPV negative disease, with median total dose of 54 Gy (IQR: 50.4–55.0 Gy) and 54 Gy (IQR: 50.4–54.0 Gy), respectively. Supplementary Figure S2 shows the time trends of HPV testing and HPV positivity in the cohort, stratified by sex and disease stage.
In the unmatched cohort, patients with HPV positive disease had better OS (log-rank p < .001), as shown in and Supplementary Table S2. Propensity score matching was performed to account for the nonrandom distribution of confounders when determining the impact of HPV status on OS, where 2103 patients with HPV positive disease were matched with 2103 patients with HPV negative disease. The baseline characteristics in the matched cohort are presented in . In the matched cohort, patients with HPV positive disease had improved OS compared to those with HPV negative disease (log-rank p = .025). Importantly, we found that for patients with early stage disease there was no difference in OS (log-rank p = .74), whereas for patients with locally advanced disease the difference in OS between patients with HPV positive disease and HPV negative disease was highly significant (log-rank p = .004), cf. .
Figure 1. Kaplan–Meier curves showing the difference in overall survival between HPV positive and negative patients with early compared to locally advanced disease.
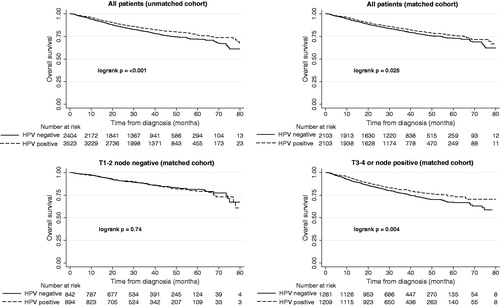
Table 2. Patient characteristics in the propensity-matched cohort.
For patients with locally advanced disease in the matched cohort, the 3-, 4- and 5-year OS were 77.1%, 71.0% and 67.3% for those who had HPV negative disease, compared to 81.1%, 76.9% and 74.1% for those with HPV positive disease (p < .03 for each time point). This finding was further confirmed by multivariable Cox proportional hazards regression as shown in , where patients with HPV positive disease had significantly better OS in the cohort with locally advanced disease (HR = 0.81, p = .018). Conversely, there was no significant difference in OS based on HPV status in patients with early stage disease (HR = 1.11, p = .43), and when examining all patients in the propensity matched cohort together there was a borderline significant OS benefit for patients with HPV positive disease (HR = 0.87, p = .059) as shown in Supplementary Table S3.
Table 3. Multivariable Cox proportional hazards regression for overall survival in the matched cohort.
We further examined whether there was a significant association between total radiation dose and OS, which was analyzed in the unmatched cohort. As shown in , there was a significant association between higher radiation dose and improved OS only in patients with locally advanced, HPV negative disease (p < .001). Interestingly there was no significant survival benefit (p > .05) in the highest dose category (≥60 Gy) as compared to the lowest dose category (30–45 Gy), in either patient sub-group.
Table 4. Multivariable Cox regression analysis showing the independent association between overall survival and total radiation dose.
We performed a sensitivity analysis to ensure that our definition of HPV positive disease did not affect the association with OS. We found that there was no difference in OS between our definition of HPV positive disease compared to excluding low-risk HPV types (log-rank p = .65) or excluding low-risk and unknown HPV types (log-rank p = .40).
Discussion
Our study utilizing the largest cohort of anal cancers with HPV tumor status to our knowledge demonstrated that patients with HPV positive anal cancers had improved OS compared to patients with HPV negative anal cancers. After propensity score matching, this benefit in OS was most pronounced in the locally advanced anal cancers with T3–T4 or node positive disease but not in the early stage anal cancers. We also showed that there was a significant improvement in OS with increasing radiation dose up to 55 Gy for patients with locally advanced, HPV negative disease, potentially motivating dose escalation efforts in these patients. This is in line with the results reported by Johnsson et al. who showed that there is a strong dose-response relationship for local tumor control in anal cancer patients with intermediate (4.0–7.9 cm) or large tumors (>7.9 cm), but not for those with small tumors [Citation8]. Importantly, we found that doses ≥60 Gy did not show any OS benefit, which may be due to increased toxicity at these very high dose levels.
Outcomes for early stage anal cancer patients were excellent in both the HPV positive and negative matched cohorts with 5-year OS of 79.3% and 81.5%, respectively. As these patients received a median total radiation dose of 50.4 Gy (IQR: 50.0–54 Gy) with 71% of patients receiving multi-agent chemotherapy, it is likely that cancer control rates were high in both cohorts and we could not detect small differences in outcomes between HPV positive and HPV negative anal cancers given excellent control rates for the entire cohort. This is contrary to the more advanced anal cancer where lack of cancer control can impact patients’ survival. This effect is further highlighted when looking at total radiation dose delivered where there was only a significant improvement in OS with increasing radiation dose for patients with poor prognosis, compared to those with HPV positive or early stage disease.
Our data are consistent with the results of the Accord 03 trial, which could not demonstrate a benefit for escalating dose beyond 60 Gy, and the single arm RTOG 92-08 could not prove a benefit to increasing the dose beyond 50 Gy [Citation6]. Furthermore, patients treated with doses above 60 Gy appear to have an increased risk for death and this may be related to increased toxicity or secondary to selection bias where those with more aggressive disease were given higher doses of radiation therapy.
Similar to other studies on the association of HPV with anal cancer, HPV-16 was the most common high-risk subtype associated with anal cancer where it was present in 76% of cases with known HPV subtype. However, HPV was only present in 59.4% of anal cancers in this cohort which is lower than that reported in the literature where it ranges from 68 to 97% [Citation9–12]. As seen in there was some selection bias of which patients had their tumors tested for HPV as it is not currently routine to test for HPV in anal cancers and only 18% of all patients in the database had HPV status reported, and only 51% of patients with known information on HPV testing actually had the test performed.
P16 immunohistochemistry could potentially be used as a surrogate for HPV testing as it would be inexpensive and can easily be done on frozen paraffin-embedded sections that are routinely prepared in most pathology laboratories. As seen in the analysis by Serup-Hansen et al. [Citation10], p16 testing had good conformity with HPV with a sensitivity of 0.95 and specificity of 0.90. It is possible that p16 may overestimate the HPV16 positivity as other HPV genotypes can also result in elevated p16 levels. Nevertheless, in our analysis, the results were not influenced by the specific HPV subtypes used in the definition of HPV positivity.
A limiting factor of this study is the fact that most patients did not have HPV status available, which makes it difficult to determine the true rate of HPV positivity in this population. Given the large spread of HPV positivity found in the literature, which also appears to depend on region, a key knowledge gap for studies of anal cancer in the USA is determining the true prevalence. In fact, there may be some under-reporting of HPV positive cases in our cohort as compared to for example the study by Serup-Hansen et al. [Citation10] This is supported by the fact that the 5-year OS found in our cohort (76%) is very similar to that of their study (74%) for HPV positive cases, whereas for HPV negative cases it is much higher in our cohort (72% vs. 52%). This suggests that an under-reporting of HPV positive cases may dilute the effect size seen in our study for the prognostic value of HPV status. Given that we still found a significant difference, especially for locally advanced disease, this highlights the need to prospectively test HPV in coming trials of anal cancer, both to determine the true prevalence of HPV positivity but also to rigorously determine its prognostic value as a biomarker.
Further limitations of this study include its retrospective nature and possibility of selection bias in the types of patients who had HPV testing, as well as the relatively low proportion of patients who had available information on tumor HPV status. Selection bias can significantly influence the assignment of patients with HPV positive or negative disease to certain treatment. We performed a propensity score-matching to account for this imbalance in the main survival analysis. Data regarding loco-regional control, cause-specific survival and functional outcomes were not recorded in this database making it difficult to determine specific oncologic and quality of life outcomes.
In conclusion, our study suggests that HPV can significantly predict for OS, especially in patients with locally advanced anal cancer. There was a benefit for dose escalation up to 55–60 Gy in patients with locally advanced, HPV negative disease. This supports the potential use of HPV testing of anal cancer tumors, especially in those with locally advanced disease, and encourages the exploitation of HPV status as a biomarker for treatment stratification.
Supplemental Material
Download MS Word (151.3 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
- Ajani JA, Winter KA, Gunderson LL, et al. Fluorouracil, mitomycin, and radiotherapy vs. fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299:1914–1921.
- Kachnic LA, Winter K, Myerson RJ, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86:27–33.
- Garg MK, Zhao F, Sparano JA, et al. Cetuximab plus chemoradiotherapy in immunocompetent patients with anal carcinoma: a Phase II Eastern Cooperative Oncology Group-American College of Radiology Imaging Network Cancer Research Group Trial (E3205). J Clin Oncol. 2017;35:718–726.
- Nigro ND, Vaitkevicius VK, Considine B, Jr. Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum. 1974;17:354–356.
- Konski A, Garcia M, Jr., John M, et al. Evaluation of planned treatment breaks during radiation therapy for anal cancer: update of RTOG 92-08. Int J Radiat Oncol Biol Phys. 2008;72:114–118.
- Peiffert D, Tournier-Rangeard L, Gerard JP, et al. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: final analysis of the randomized UNICANCER ACCORD 03 trial. J Clin Oncol. 2012;30:1941–1948.
- Johnsson A, Leon O, Gunnlaugsson A, et al. Determinants for local tumour control probability after radiotherapy of anal cancer. Radiother Oncol. 2018;128:380–386.
- Frisch M, Glimelius B, van den Brule AJ, et al. Sexually transmitted infection as a cause of anal cancer. N Engl J Med. 1997;337:1350–1358.
- Serup-Hansen E, Linnemann D, Skovrider-Ruminski W, et al. Human papillomavirus genotyping and p16 expression as prognostic factors for patients with American Joint Committee on Cancer stages I to III carcinoma of the anal canal. J Clin Oncol. 2014;32:1812–1817.
- Alemany L, Saunier M, Alvarado-Cabrero I, et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer. 2015;136:98–107.
- Mai S, Welzel G, Ottstadt M, et al. Prognostic relevance of HPV infection and p16 overexpression in squamous cell anal cancer. Int J Radiat Oncol Biol Phys. 2015;93:819–827.
- Jhaveri J, Rayfield L, Liu Y, et al. Prognostic relevance of human papillomavirus infection in anal squamous cell carcinoma: analysis of the national cancer data base. J Gastrointest Oncol.
- Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35.
- Kimple RJ, Smith MA, Blitzer GC, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013;73:4791–4800.
